Preliminary results for genetic transformation of shoot tip of Eucalyptus saligna Sm. via Agrobacterium tumefaciens
DOI:
https://doi.org/10.20873/jbb.uft.cemaf.v2n1.silvaPalabras clave:
Eucaliptos, gene GUS, linhagem EHA105Resumen
The regeneration of transgenic plants of Eucalypt is the largest difficulty for the genetic transformation of this genus; in addition a low rate of transformed plants is reached. The aim of this research was to evaluate acetosyringone (3',5'-Dimethoxy-4'-hydroxyacetophenone) on the co-culture medium during genetic transformation of shoot tips of Eucalyptus saligna via Agrobacterium tumefaciens and to promote the explants selection supposedly transformed. Shoot tip from multiple shoots was used as explants. These explants were pre-cultured during two days before the transformation. Strain EHA105 of A. tumefaciens harboring the plasmid pBI121 was used. The treatments were: 0 and 100 μM acetosyringone added to the co-culture, after co-culture the explants were cultured in multiplication medium supplemented with 250 mg.L-1 Cefotaxime® and each sub-culture the kanamycin levels were increased from 50 to 150 mg.L-1. The transient expression of the uidA gene in shoot tips was evaluated after the end of the co-culture (fifth day) and after seven days of culture on medium containing kanamycin. The presence of 100 µM acetosyringone at the co-culture of shoot tips of Eucalyptus saligna promoted higher transient expression of the uidA gene and retards toxic effects caused by kanamycin.
Citas
AKAMA, K.; SHIRAISHI, H.; OHTA, S.; NAKAMURA, K.; OKADA, K.; SHIMURA, Y. (1992), Efficient transformation of Arabidopsis thaliana: comparison of the efficiencies with various organs, plant ecotypes and Agrobacterium strains. Plant Cell Reports, 12, 7-11.
BRONDANI, G. E.; DUTRA, L. F.; GROSSI, F.; WENDLING, I.; HORNIG, J. (2009), Establishment, multiplication and elongation in vitro of Eucalyptus benthamii Maiden & Cambage x Eucalyptus dunnii Maiden. Revista Árvore, 33, 11- 19.
BRONDANI, G. E.; WENDLING, I.; GROSSI, F.; DUTRA, L. F.; ARAUJO, M. A. (2010a), Eucalyptus benthamii × Eucalyptus dunnii minicutting technique: (ii) minicutting survival and rooting in relation to collection and seasons. Ciência Florestal, 20, 453- 465.
BRONDANI, G. E.; WENDLING, I.; GROSSI, F.; DUTRA, L. F.; ARAUJO, M. A. (2010b), IBA application for rooting of Eucalyptus benthamii Maiden and Cambage x Eucalyptus dunnii Maiden minicuttings. Acta Scientiarum. Agronomy, 32, 667-674.
CORRÊA, L. R.; PAIM, D. C.; SCHWAMBACH, J.; FETT- NETO, A. G. (2005), Carbohydrates as regulatory factors on the rooting of Eucalyptus saligna Smith and Eucalyptus globulus Labill. Plant Growth Regulation, 45, 63-73.
CRUZ, C. D. (2001), Programa Genes: versão Windows; aplicativo computacional em genética e estatística. Viçosa: UFV, Imprensa Universitária. p. 648.
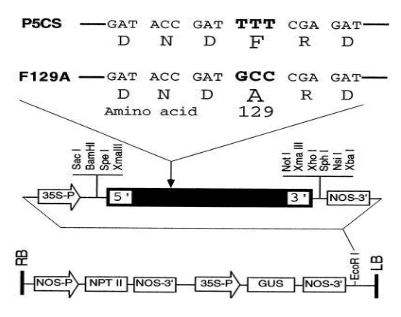
DIBAX, R.; DESCHAMPS, C.; BESPALHOK FILHO, J. C.; VIEIRA, L. G. E.; MOLINARI, H. B. C.; CAMPOS M. K. F.; QUOIRIN, M. (2010), Organogenesis and Agrobacterium tumefaciens-mediated transformation of Eucalyptus saligna with P5CS gene. Biologia Plantarum, 54, 6-12.
DIBAX, R. Transformação e expressão do gene PC5SF129-A em Eucalytus saligna. Tese (Doutorado em Agronomia) - Universidade Federal do Paraná, 2007.
DUTT, M.; LEE, D. H.; GROSSER, J. W. (2010), Bifunctional selection–reporter systems for genetic transformation of citrus: mannose- and kanamycin-based systems. In Vitro Cellular & Developmental Biology - Plant, 46, 467-476.
FERRAZ, E. S. B. AND COUTINHO, A. R. (1984), Efeitos da geada na madeira de Eucalyptus saligna. IPEF, 28, 57-62.
GRAAFF, E. E.; AUER, C. A.; HOOYKAAS, P. J. J. (2001), Altered development of Arabidopsis thaliana carrying the Agrobacterium tumefaciens ipt gene is partially due to ethylene effects. Plant Growth Regulation, 34, 305-315.
HONG, Z.; LAKKINENI, K.; ZHANG, Z.; VERMA, D. P. S. (2000), Removal of feedback inhibition of 1- Pyrroline-5-Carboxylate Synthetase results in increased Proline accumulation and protection of plants from osmotic stress. Plant Physiology, 122, 1129-1136.
HOOD, E. E.; GELVIN, S. B.; MELCHERS, L. S.; HOEKEMA, A. (1993), New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Resource, 2, 208-218.
JEFFERSON, R. A.; KAVANAGH, T. A.; BEVAN, M. W. (1987), GUS fusions beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal, 6, 3901-3907.
KARAVAIKO, N. N.; SELIVANKINA, S. Y. U.; KUDRYAKOVA, N. V.; MASLOVA, G. G.; BURKHANOVA, E. A.; ZUBKOVA, N. K.; KULAEVA, O. N. (2004), Is a 67-kD cytokinin-binding protein from barley and Arabidopsis thaliana leaves involved in the leaf responses to phenylurea derivatives? (A Review). Russian Journal of Plant Physiology, 51, 790-797.
LE ROUX, J. J. AND VAN STADEN, J. (1991), Micropropagation of Eucalyptus species. Hortscience, 26, 199-200.
MURASHIGE, T. AND SKOOG, F. (1962), Revised medium for rapid growth and bioassays with tobacco tissue culture. Physiologia Plantarum, 15, 473-497.
SELLE, G. L. AND VUADEN, E. (2008), Efeitos da geada sobre plantações de Eucalypus grandis. Caderno de Pesquisa Série Biologia, 20, 36-44.
SILVA, A. L. L.; WALTER, J. M.; HORBACH, M. A.; QUOIRIN, M. (2007), Contenção do fluxo gênico de plantas geneticamente modificadas. Caderno de Pesquisa Série Biologia, 19, 18-26.
SILVA, A. L. L.; OLIVEIRA, Y.; ALCANTARA, G. B.; SANTOS, M.; QUOIRIN, M. (2009), Tolerância ao resfriamento e congelamento de folhas de eucalipto. Biociências, 17, 86-90.
SILVA, A. L. L.; OLIVEIRA, Y.; COSTA, J. L.; MASETTO, E.; MUDRY, C. S.; ERASMO, E. A. L.; SCHEIDT, G. N. (2010), Shoot tip and cotyledon explants of Eucalyptus saligna Sm. cultivated on different kanamycin levels. Journal of Biotechnology and Biodiversity, 1, 1-5.
VATANKHAH, E.; NIKNAM, V.; EBRAHIMZADEH, H. (2010), Activity of antioxidant enzyme during in vitro organogenesis in Crocus sativus. Biologia Plantarum, 54, 509-514.
VERVLIET, G.; HOLSTERS, M.; TEUCHY, H.; VAN MONTAGU, M.; SCHELL, J. (1975), Characterization of different plaque-forming and defective temperate phages in Agrobacterium strains. Journal of General Virology, 23, 33-48.
WALZ, A.; SEIDEL. C.; RUSAK, G.; PARK, S.; COHEN, J. D.; LUDWIG-MÜLLER, J. (2008), Heterologous expression of IAP1, a seed protein from bean modified by indole-3-acetic acid, in Arabidopsis thaliana and Medicago truncatula. Planta, 227, 1047-1061.
ZHANG, C-S.; LU, Q.; VERMA, D. P. S. (1995), Removal of feedback inhibition of 1-Pyrroline-5- Carboxylate Synthetase, a bifunctional enzyme catalyzing the first two steps of proline biossynthesis in plants. Journal of Biological Chemistry, 270, 20491-20496.
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Copyright (c) 2024 - Journal of Biotechnology and Biodiversity

Esta obra está bajo una Licencia Creative Commons Atribución 4.0 Internacional.
Los autores que publican en esta revista aceptan los siguientes términos:
Los autores mantienen los derechos autorales y conceden a la revista el derecho de primera publicación, con el trabajo simultáneamente licenciado bajo la LicenciaCreative Commons Attribution (CC BY 4.0 en el link http://creativecommons.org/licenses/by/4.0/) que permite compartir el trabajo con reconocimiento de la autoría y publicación inicial en esta revista.
Los autores tienen autorización para asumir contratos adicionales separadamente, para distribución no exclusiva de la versión del trabajo publicado en esta revista (ej.: publicar en repositorio institucional o como capítulo de libro), con reconocimiento de autoría y publicación inicial en esta revista.
A los autores se les permite, y son estimulados, a publicar y distribuir su trabajo online (ej.: en repositorios institucionales o en su página personal) en cualquier punto antes o durante el proceso editorial, ya que esto puede generar alteraciones productivas, bien como aumentar el impacto y la citación del trabajo publicado (disponible en El Efecto del Acceso Libre en el link http://opcit.eprints.org/oacitation-biblio.html).


