Biotechnological potential of the endophytic fungi isolated from Clitoria guianensis
DOI:
https://doi.org/10.20873/jbb.uft.cemaf.v12n2.17691Palabras clave:
endosymbionts fungi, vergateza, antioxidant activity, allelopathic activityResumen
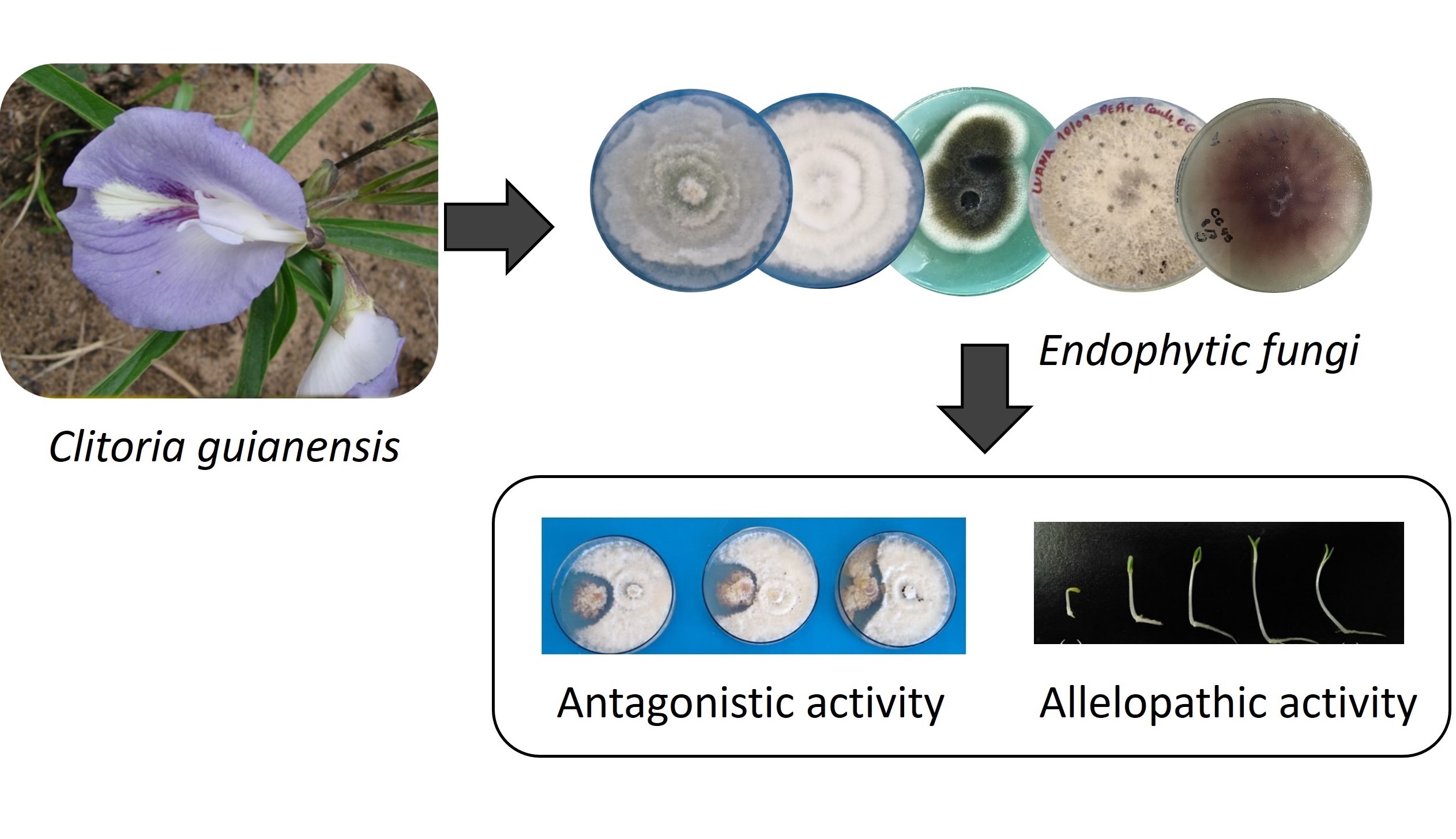
The biodiversity of endophytic fungi in the Cerrado region in the north of Brazil remains largely unexplored, however it is important to explore the biotechnological potential of these endophytes. In this study, we isolated seventeen endophytic fungi strains from the medicinal plant Clitoria guianensis and tested the ability of these strain to inhibit the phytopathogenic fungi: Fusarium oxysporum, Bipolaris oryzae, and Curvularia lunata. The in vitro antagonistic assay was evaluated on different time scales (six and twelve days), we observed that all strains of endophytic fungi, isolated from C. guianenses, inhibited the growth of F. oxysporum and B. oryzae after six days, for C. lunata just ten strains inhibited its growth in the first six days. In twelve days, only the strains CGF1, CGF3, and CGF4 inhibited the growth of F. oxysporum, while for the other phytopathogenic fungi the inhibitions were not observed. The endophytic fungi were cultivated in a controlled environment using potato dextrose broth (PDB) medium and the crude extracts were obtained by extracting the fungi culture with ethyl acetate (EtOAc). All the crude extracts exhibited antioxidant activity, as assessed by the 2,2 diphenyl-1-picrylhydrazyl (DPPH) method. Additionally, four different concentrations (C100, C500, C1000 and C2000) of the crude extracts were evaluated in the in vitro allelopathic assay using Lactuca sativa (lettuce) seeds. Some fungal extracts demonstrated allelopathic effects by inhibiting the growth of Lactuca sativa seedlings, for example, the crude extract obtained from the strain CGF7 at a concentration of C2000 inhibited radicle development by 75.2%. While others acted as growth promoters, as is the case with the crude extract of the CGF10 strain, which contributed to lettuce radicle growth by 15.9% in C2000. Overall, these findings suggest that endophytic fungi associated with C. guianensis have significant biotechnological potential and can be used as biocontrol agents against phytopathogens and specific plants, in addition to growth promoters, making them valuable tools for sustainable agriculture.
Citas
Araujo WL, Lacava PT, Marcon J, Lima AOS, Sobral JK, Pizzirani-Kleiner AA, Azevedo JL. Guia prático: isolamento e caracterização de microrganismos endofíticos. Piracicaba: CALO, p.167, 2010.
Behie SW, Bidochka JM. Potential agricultural benefits through biotechnological manipulation of plant fungal asso-ciations. BioEssays, v. 35, n. 4, p. 328–331, 2013. https://doi.org/10.1002/bies.201200147
Bell DK, Wells HD, Markham CR. In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathology, v. 72, p. 379–82, 1982.
Chapla VM, Biasetto CR, Araujo AR. Endophytic fungi: An unexplored and sustainable source of new and bioactive nat-ural products. Revista Virtual de Química, v. 5, n. 3, p. 421–437, 2013.
https://doi.org/10.5935/1984-6835.20130036.
Chen JL, Shi ZS, Cui PM, Kai W, Chen YW et al. Endophytic Trichoderma gamsii YIM PH30019: A promising biocon-trol agent with hyperosmolar, mycoparasitism, and antago-nistic activities of induced volatile organic compounds on root-rot pathogenic fungi of Panax notoginseng. Journal of Ginseng Research, v. 40, n. 4, p. 315–324, 2016. https://doi.org/10.1016/j.jgr.2015.09.006.
Cunha CL, Siebeneichler SC, Nascimento IR, Holzbach JC. A New Isoflavone and Other Constituents from Roots of Cli-toria guianensis. Journal of the Brazilian Chemical Society, v. 31, n. 8, p. 1753–1757, 2020. https://doi.org/10.21577/0103-5053.20200061.
Dai CC, Gao FK, Liu, XZ. Mechanisms of fungal endophytes in plant protection against pathogens. African Journal of Microbiology Research, v. 4, n.13, p. 1346–51, 2010.
Dantas SBS, Alves FAM, Chapla VM. Chemical, diversity, and biotechnological potential of endophytic fungi isolated from Brazilian Cerrado plants. Biota Neotropica, v. 21, n. 2, 2021.
https://doi.org/10.1590/1676-0611-bn-2020-1069
Ferreira DF Sisvar: a guide for its bootstrap procedure in multiple comparisons. Ciência e Agrotecnologia, v. 38, p. 109-112, 2014.
Francisco ASS, Carlos, AVZ. The Assistat Software Version 7.7 and its use in the analysis of experimental data. African Journal of Agricultural Research, v. 11, n. 39, p. 3733–40, 2016.
doi: 10.5897/ajar2016.11522.
Grabka R, D’entremont TW, Adam, SJ, Walker AK, Tanney JB, Abbasi PA, Ali S. Fungal Endophytes and Their Role in Agricultural Plant Protection against Pests and Pathogens. Plants, v. 11, n. 3, p. 384, 2022. https://doi.org/10.3390/plants11030384
Hamzah TNT, Lee SY, Hidayat A, Terhem R, Faridah-Hanum I, Mohamed R. Diversity and characterization of endophytic fungi isolated from the tropical mangrove species, Rhi-zophora mucronata, and identification of potential antago-nists against the soil-borne fungus, Fusarium solani. Fron-tiers in Microbiology, v. 15, n. 9, p. 1705, 2018. https://doi.org/10.3389/fmicb.2018.01707.
Huang LQ, Ni, Y, Su L, Deng H, Lyu H. The potential of endophytic fungi isolated from cucurbit plants for biocontrol of soilborne fungal diseases of cucumber. Microbiological Research, v. 231, 2020.
https://doi.org/10.1016/j.micres.2019.126369
Huang WY, Cai YZ, Hyde KD, Corke H, Sun M. Endophytic fungi from Nerium oleander L (Apocynaceae): Main con-stituents and antioxidant activity. World Journal of Microbi-ology and Biotechnology, v. 23, n. 9, p. 1253–63, 2007. doi: 10.1007/s11274-007-9357-z.
Khamare Y, Chen J, Marble SC. Allelopathy and its appli-cation as a weed management tool: A review. Frontiers in Plant Science, v. 28, n. 13, p. 1034649, 2022. https://doi.org/10.3389/fpls.2022.1034649.
Khare E, Mishra J, Arora NK. Multifaceted interactions be-tween endophytes and plant: Developments and Prospects. Frontiers in Microbiology, v. 15, n. 9, p. 2732, 2018. https://doi.org/10.3389/fmicb.2018.02732.
Latif S, Chiapusio G, Weston LA. Allelopathy and the Role of Allelochemicals in Plant Defence. Advances in Botanical Research, v. 82, p. 19–54, 2017. https://doi.org/10.1016/bs.abr.2016.12.001.
Llauradó MG, Rodríguez DM, Hendrix S, Arranz JCE, Boix YF, Pacheco AO, Díaz JG, Quevedo HJM, Dubois AF, Aleman EI, Beenaerts N, Santos IEM, Ratón TO, Cos P, Cuypers A. Antioxidants in plants: A valorization potential emphasizing the need for the conservation of plant biodiver-sity in Cuba. Antioxidants, v. 9, n. 11, p. 1–39, 2020. doi: 10.3390/antiox9111048.
Lopes JC, Chagas Junior AF, Neves ACC, Chapla VM, Batistella AR. Fungos endofíticos isolados do capim citro-nela e seleção de antagonistas a fitopatógenos. Biota Ama-zônica, v. 7, n. 3, p. 84–88, 2017.
https://doi.org/10.18561/2179-5746/biotaamazonia.v7n3p84-88
Manganyi MC, Ateba CN. Untapped potentials of endophytic fungi: A review of novel bioactive compounds with biologi-cal applications. Microorganisms, v. 8, n. 12, p.1934, 2020.
https://doi.org/10.3390/microorganisms8121934.
Mishra A, Surendra KG, Kumar A, Sharma VK, Verma SK, Kharwar RN, Sieber TN. Season and Tissue Type Affect Fungal Endophyte Communities of the Indian Medicinal Plant Tinospora cordifolia More Strongly than Geographic Location. Microbial Ecology, v. 64, n. 2, p. 388–98, 2012. doi: 10.1007/s00248-012-0029-7.
Oliveira RJV, Sousa NMF, Pinto Neto WPP, Bezerra JL, Silva GA, Cavalcanti, MAQ. Seasonality affects the com-munity of endophytic fungi in coconut (Cocos nucifera) crop leaves. Acta Botanica Brasilica, v. 34, n. 4, p. 704–11, 2020.
doi: 10.1590/0102-33062020abb0106.
Pereira JC, Paulino CLA, Endres L, Santana AEG, Pereira FRS, Souza RC. Allelopathic potential of ethanolic extract and phytochemical analysis of Paspalum maritimum trind. Planta Daninha, v. 37, 2019. https://doi.org/10.1590/S0100-83582019370100053
Rai M, Rathod D, Agarkar G, Dar M, Brestic M, Pastore GM, Marostica Junior MR. Fungal growth promotor endophytes: A pragmatic approach towards sustainable food and agricul-ture. Symbiosis, v. 62, n. 2, p. 63–79, 2014. https://doi.org/10.1007/s13199-014-0273-3.
Ribeiro BA, Mata TB, Canuto GAB, Silva EO. Chemical Diversity of Secondary Metabolites Produced by Brazilian Endophytic Fungi. Current Microbiology, v. 78, n. 1, p. 33–54, 2021.
https://doi.org/10.1007/s00284-020-02264-0
Sadeghi F, Samsampour D, Seyahooei MA, Bagheri A, Soltani J. Diversity and Spatiotemporal Distribution of Fun-gal Endophytes Associated with Citrus reticulata cv. Si-yahoo. Current Microbiology, v. 76, n. 3, p. 279–89, 2019.
doi: 10.1007/s00284-019-01632-9.
Santos CM, Ribeiro AS, Garcia A, Polli AD, Polonio JC, Azevedo JL, Pamphile JÁ. Enzymatic and antagonist activi-ty of endophytic fungi from Sapindus saponaria L. (Sapin-daceae). Acta Biológica Colombiana, v. 24, n. 2, p. 322–30, 2019.
doi: 10.15446/abc.
Santos TT, Varavallo MA. Aplicação de microrganismos endofíticos na agricultura e na produção de substâncias de interesse econômico. Semina: Ciências Biológicas e da Saú-de, v. 32, n. 2, p. 199–212, 2011.
doi: 10.5433/1679-0367.2011v32n2p199.
Silva-Valderrama I, Toapanta D, Miccono MA, Lolas M, Díaz GA, Cantu D, Castro A. Biocontrol potential of Grapevine endophytic and Rhizospheric Fungi against trunk pathogens. Frontiers in Microbiology, v. 11, p. 1–13, 2021.
doi: 10.3389/fmicb.2020.614620.
Simões-Pires CA., Queiroz EF, Henriques AT, Hostettmann K. Isolation and on-line identification of antioxidant com-pounds from three Baccharis species by HPLC-UV-MS/MS with post-column derivatization. Phytochemical Analysis, v. 16, p. 307–14, 2005. https://doi.org/10.1002.pca.826.
Singh DK, Sharma VK, Kumar J, Mishra A, Verma SK, Sieber TN, Kharwar RN. Diversity of endophytic mycobiota of tropical tree Tectona grandis Linn.f.: Spatiotemporal and tissue type effects. Scientific Reports, v. 7, n. 1, 2017. doi: 10.1038/s41598-017-03933-0.
Soares DF. Análise química e biológica dos extratos brutos, hexânico e acetato de etila obtidos das folhas da espécie Cli-toria guianensis Benth. 2016. Monografia (Química Ambi-ental). Universidade Federal do Tocantins, Gurupi.
Souza BS, Santos TT. Endophytic fungi in economically important plants: ecological aspects, diversity and potential biotechnological applications. Journal of bioenergy and food Science, v. 4, n. 2, p. 113–26, 2017.
doi: 10.18067/jbfs.v4i2.121.
Souza CD, Felfili JM. Uso de plantas medicinais na região de Alto Paraíso de Goiás, GO, Brasil. Acta Botânica Brasilica, v. 20, p. 135–142, 2006.
Strobel G. The emergence of endophytic microbes and their biological promise. Journal of Fungi, v. 4, n. 2, 2018.
doi: 10.3390/jof4020057.
Vila Verde GM, Paula JR, Carneiro DM. Levantamento et-nobotânico das plantas medicinais do cerrado utilizadas pela população de Mossâmedes (GO). Revista Brasileira de Farmacognosia, v. 13, p. 64–66, 2003.
Yan L, Zhu J, Zhao X, Shi J, Jiang C, Shao G. Beneficial effects of endophytic fungi colonization on plants. Applied Microbiology and Biotechnology, v. 103, n. 8, p. 3327–40, 2019.
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2024 Luana Fernandes Ferraz, Luiz Renato Lima Silva Miranda, Gleys Kellen Aquino Moraes, Aloísio Freitas Chagas Junior, Vanessa Mara Chapla

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Copyright (c) 2024 - Journal of Biotechnology and Biodiversity

Esta obra está bajo una Licencia Creative Commons Atribución 4.0 Internacional.
Los autores que publican en esta revista aceptan los siguientes términos:
Los autores mantienen los derechos autorales y conceden a la revista el derecho de primera publicación, con el trabajo simultáneamente licenciado bajo la LicenciaCreative Commons Attribution (CC BY 4.0 en el link http://creativecommons.org/licenses/by/4.0/) que permite compartir el trabajo con reconocimiento de la autoría y publicación inicial en esta revista.
Los autores tienen autorización para asumir contratos adicionales separadamente, para distribución no exclusiva de la versión del trabajo publicado en esta revista (ej.: publicar en repositorio institucional o como capítulo de libro), con reconocimiento de autoría y publicación inicial en esta revista.
A los autores se les permite, y son estimulados, a publicar y distribuir su trabajo online (ej.: en repositorios institucionales o en su página personal) en cualquier punto antes o durante el proceso editorial, ya que esto puede generar alteraciones productivas, bien como aumentar el impacto y la citación del trabajo publicado (disponible en El Efecto del Acceso Libre en el link http://opcit.eprints.org/oacitation-biblio.html).


