Behavior of the conversion of glucose, galactose and lactose to lactic acid by kefir grains
DOI:
https://doi.org/10.20873/jbb.uft.cemaf.v11n1.aguiarPalabras clave:
lactic acid bacteria, mixture culture, primary metabolism, fermentationResumen
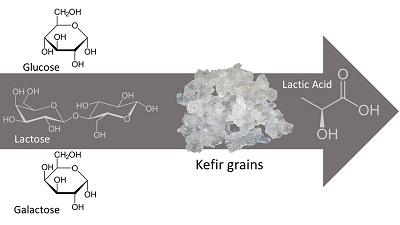
Few studies have been reported on lactic acid production by kefir grains. Kefir has been widely associated with probiotic use due to its cell growth in food matrices such as milks, juices and sugary solutions. However, from industrial scale there are no reports of its use in lactic acid production. In this work, we carried out experiments to test and understand how glucose, galactose and lactose, during lactic-acid fermentation, were converted in lactic acid by kefir grains. Given the microbial complexity in coexistence in kefir grains, it is likely that kinetic studies are not the most appropriate for the positive or negative definition of the kefir use as a starter in the lactic acid fermentative production on an industrial scale. It was concluded that, although with a higher Lag phase, lactose was the substrate that best presented a product and cell conversion rate, although glucose and galactose can also be used as a substrate in the production of this carboxylic acid.
Citas
Abdel-Rahman MA, Hassan SED, Alrefaey HMA, El-Belely EF, Elsakhawy T, Fouda A, Khattab SMR. Subsequent im-provement of lactic acid production from beet molasses by Enterococcus hirae ds10 using different fermentation strate-gies. Bioresource Technology Reports, v.13, 2021. https://doi.org/10.1016/j.biteb.2020.100617
Abedi E, Hashemi SMB. Lactic acid production - producing microorganisms and substrates sources-state of art. Heliyon, v.6, n.10, p.1-32, 2020. https://doi.org/10.1016/j.heliyon.2020.e04974
Ahmad A, Banat F, Taher H. A review on the lactic acid fer-mentation from low-cost renewable materials: Recent deve-lopments and challenges. Environmental Technology & In-novation, v.20, p.1-21, 2020. https://doi.org/10.1016/j.eti.2020.101138
Aso Y, Hashimoto A, Ohara H. Engineering Lactococcus lactis for D-lactic acid production from starch. Current Mi-crobiology, v.76, p.1186-1192, 2019. https://doi.org/10.1007/s00284-019-01742-4
Borshchevskaya LN, Gordeeva TL, Kalinina AN, Sineokii SP. Spectrophotometric determination of lactic acid. Journal of Analytical Chemistry, v.71, p.755-758, 2016.
Bulut S, Elibol M, Ozer D. Effect of different carbon sources on L(+)-lactic acid production by Rhizopus oryzae. Bioche-mical Engineering Journal, v.21, n.1, p.33-37, 2004.
Cock LS, Stouvenel AR. Biotechnological production of lactic acid: state of the art. CYTA-Journal of Food, v.5, n.1, p.54-65, 2005.
Cui Y, Ning M, Chen H, Zeng X, Yue Y, Yuan Y, Yue T. Microbial diversity associated with Tibetan kefir grains and its protective effects against ethanol-induced oxidative stress in HepG2 cells. Food Bioscience, v.50, p.1-12, 2022. https://doi.org/10.1016/j.fbio.2022.102151
Corrieu G, Béal C. Yogurt: the product and its manufacture. Encyclopedia of Food and Health, p.617-624, 2016. https://doi.org/10.1016/b978-0-12-384947-2.00766-2
Dillirani N, Oktarina N, Chen PT, Chen CY, Lee DJ, Chang JS. Fermentative lactic acid production from seaweed hydro-lysate using Lactobacillus sp. and Weissella sp. Bioresource Technology, v.344, p.1-9, 2022. https://doi.org/10.1016/j.biortech.2021.126166
González-Leos A, Bustos M, Rodríguez-Castillejos G, Durán LR, Ángel J. Kinetics of lactic acid fermentation from sugar-cane bagasse by Lactobacillus pentosus. Revista Mexicana de Ingeniería Química, v.19, n.1, p.377-386, 2019. https://doi.org/10.24275/rmiq/Alim618
Hassan SED Abdel-Rahman, MA, Roushdy MM, Azab MS, Gaber MA. Effective biorefinery approach for lactic acid production based on co-fermentation of mixed organic was-tes by Enterococcus durans BP130. Biocatalysis and Agri-cultural Biotechnology, v.20, p.1-9, 2019. https://doi.org/10.1016/j.bcab.2019.101203
Hofvendahl K, Hahn-Hägerdal B. Factors affecting the fer-mentative lactic acid production from renewable resources. Enzyme Microbiology and Technology, v.26, n.2-4, p.87-107, 2000. https://doi.org/10.1016/s0141-0229(99)00155-6
Iskandar CF, Cailliez-Grimal C, Borges F, Revol-Junelles AM. Review of lactose and galactose metabolism in Lactic Acid Bacteria dedicated to expert genomic annotation. Trends in Food Science & Technology, v.88, p.121-132, 2019. https://doi.org/10.1016/j.tifs.2019.03.020
Jeckelmann JM, Erni B. Transporters of glucose and other carbohydrates in bacteria. Pflügers Archiv - European Jour-nal of Physiology, v.472, n.9, p.1129-1153, 2020. https://doi.org/10.1007/s00424-020-02379-0
Karolenko CE, Wilkinson J, Muriana PM. Evaluation of vari-ous lactic acid bacteria and generic E. coli as potential non-pathogenic surrogates for in-plant validation of biltong dried beef processing. Microorganisms, v.10, n.8, p.1648, 2022. https://doi.org/10.3390/microorganisms10081648
Kleyn DH. Determination of lactose by an enzymatic method. Journal of Dairy Science, v.68, n.10, p.2791-2798, 1985. https://doi.org/10.3168/jds.s0022-0302(85)81167-x
Kobayashi T, Kajiwara M, Wahyuni M, Hamada‐Sato N, Imada C, Watanabe E. Effect of culture conditions on lactic acid production of Tetragenococcus species. Journal of Ap-plied Microbiology, v.96, n.6, p.1215-1221, 2004. https://doi.org/10.1111/j.1365-2672.2004.02267.x
Kotzamanidis C, Roukas T, Skaracis G. Optimization of lactic acid production from beet molasses by Lactobacillus del-brueckii NCIMB 8130. World Journal of Microbiology and Biotechnology, v.18, p.441-448, 2002. https://doi.org/10.1023/A:1015523126741
Lazarova Z, Peeva L. Solvent extraction of lactic acid from aqueous solution, v.32, n.1, p.75-82, 1994. https://doi.org/10.1016/0168-1656(94)90122-8
Liu J, Piao H, Liu C. Characterization of key enzymes for D-lactic acid synthesis in Leuconostoc citreum KM20. Bio-technology and Bioprocess, v.27, p.921-929, 2022. https://doi.org/10.1007/s12257-022-0110-0
Magalhães KT, Dragone G, Pereira GVM, Oliveira JM, Do-mingues L, Teixeira JA, Silva JBA, Schwan RF. Compara-tive study of the biochemical changes and volatile compound formations during the production of novel whey-based kefir beverages and traditional milk kefir. Food Chemistry, v.126, n.1, p.249-253, 2011b. https://doi.org/10.1016/j.foodchem.2010.11.012
Magalhães KT, Pereira GVDM, Campos CR, Dragone G, Schwan RF. Brazilian kefir: structure, microbial communi-ties and chemical composition. Brazilian Journal of Micro-biology, v.42, p.693-702, 2011a.
Malik M, Bora J, Sharma V. Growth studies of potentially probiotic lactic acid bacteria (Lactobacillus plantarum, Lac-tobacillus acidophilus, and Lactobacillus casei) in carrot and beetroot juice substrates. Journal of Food Processing and Preservation, v.43, n.11, p.1-8, 2019. https://doi.org/10.1111/jfpp.14214
Montero-Zamora J, Fernández-Fernández S, Redondo-Solano M, Mazón-Villegas B, Mora-Villalobos JA, Barboza N. Assessment of different lactic acid bacteria isolated from agro-industrial residues: first report of the potential role of Weissella soli for lactic acid production from milk whey. Applied Microbiology, v.2, n.3, p.626-635, 2022. https://doi.org/10.3390/applmicrobiol2030048
Mostefa N, Abid A, Boumédiène KM. Preliminary probiotic potential of selected Aerococcus spp., Enterococcus spp., and Weisella sp. from Algerian guedid. Journal of Micro-biology, Biotechnology and Food Sciences, v.10, n.6, p.1-6, 2021. https://doi.org/10.15414/jmbfs.2937
Olszewska-Widdrat A, Alexandri M, López-Gómez JP, Schneider R, Venus J. Batch and continuous lactic acid Fermentation based on a multi-substrate approach. Microor-ganisms, v.8, n.7, p.1-14, 2020. https://doi.org/10.3390/microorganisms8071084
Pessione E. Lactic acid bacteria contribution to gut microbiota complexity: lights and shadows. Frontiers in Cellular and Infection Microbiology, v.2, p.1-15, 2012. https://doi.org/10.3389/fcimb.2012.00086
Qiu Z, Fang C, Gao Q, Bao J. A short-chain dehydrogenase plays a key role in cellulosic D-lactic acid fermentability of Pediococcus acidilactici. Bioresource Technology, v.297, p.1-7, 2020. https://doi.org/10.1016/j.biortech.2019.122473
Rawoof SAA, Kumar PS, Vo DVN. Production of optically pure lactic acid by microbial fermentation: a review. Envi-ronmental Chemistry Letters, v.19, p.539-556, 2021. https://doi.org/10.1007/s10311-020-01083-w
Ringø E, Doan HV, Lee S, Song SK. Lactic acid bacteria in shellfish: possibilities and challenges. Reviews in Fisheries Science & Aquaculture, v.28, n.2, p.139-169, 2020. https://doi.org/10.1080/23308249.2019.1683151
Song YR, Lee CM, Lee SH, Baik SH. Evaluation of probiotic properties of Pediococcus acidilactici M76 producing func-tional exopolysaccharides and its lactic acid fermentation of black raspberry extract. Microorganisms, v.9, n.7, p.1-17, 2021. https://doi.org/10.3390/microorganisms9071364
Sudhakar MP, Dharani G. Evaluation of seaweed for the production of lactic acid by fermentation using Lactobacillus plantarum. Bioresource Technology Reports, v.17, p.1-5, 2022. https://doi.org/10.1016/j.biteb.2021.100890
Taye Y, Degu T, Fesseha H, Mathewos M. Isolation and identification of lactic acid bacteria from cow milk and milk products. The Scientific World Journal, v.2021, p.1-6, 2021. https://doi.org/10.1155/2021/4697445
Tsouli SS, Belkhou R, Bouseta A, Hayaloglu AA. Evaluation of techno-functional and biochemical characteristics of selec-ted lactic acid bacteria (Lactococcus lactis subsp. lactis and Leuconostoc mesenteroides subsp. mesenteroides) used for the production of Moroccan fermented milk. International Dairy Journal, v.140, p.1-10, 2023. https://doi.org/10.1016/j.idairyj.2023.105592
Wang Y, Chan KL, Abdel-Rahman MA, Sonomoto K, Leu SY. Dynamic simulation of continuous mixed sugar fermen-tation with increasing cell retention time for lactic acid pro-duction using Enterococcus mundtii QU 25. Biotechnology for Biofuels, v.13, n.112, p.1-16, 2020. https://doi.org/10.1186/s13068-020-01752-6
Wu Y, Li S, Tao Y, Li D, Han Y, Show PL, Zhou J. Fermen-tation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacte-rium bifidum: Growth of probiotics, metabolism of pheno-lics, antioxidant capacity in vitro and sensory evaluation. Food Chemistry, v.348, n.30, p.1-16, 2021. https://doi.org/10.1016/j.foodchem.2021.129083
Yang Q, Lü Y, Zhang M, Gong Y, Li Z, Tran NT, Li S. Lactic acid bacteria, Enterococcus faecalis Y17 and Pediococcus pentosaceus G11, improved growth performance, and immunity of mud crab (Scylla paramamosain). Fish & Shellfish Immunology, v.93, p.135-143, 2019. https://doi.org/10.1016/j.fsi.2019.07.050
Yu P, Li N, Geng M, Liu Z, Liu X, Zhang H, Chen W. Short communication: Lactose utilization of Streptococcus ther-mophilus and correlations with β-galactosidase and urease. Journal of Dairy Science, v.103, n.1, p.166-171, 2019. https://doi.org/10.3168/jds.2019-17009
Zaunmüller T, Unden G. Transport of sugars and sugar alco-hols by lactic acid bacteria. Biology of Microorganisms on Grapes, in Must and in Wine, p.149-163, 2008. https://doi.org/10.1007/978-3-540-85463-0_8
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2023 Claudio Lima, Alessandra Menegazzo Rosa

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Copyright (c) 2024 - Journal of Biotechnology and Biodiversity

Esta obra está bajo una Licencia Creative Commons Atribución 4.0 Internacional.
Los autores que publican en esta revista aceptan los siguientes términos:
Los autores mantienen los derechos autorales y conceden a la revista el derecho de primera publicación, con el trabajo simultáneamente licenciado bajo la LicenciaCreative Commons Attribution (CC BY 4.0 en el link http://creativecommons.org/licenses/by/4.0/) que permite compartir el trabajo con reconocimiento de la autoría y publicación inicial en esta revista.
Los autores tienen autorización para asumir contratos adicionales separadamente, para distribución no exclusiva de la versión del trabajo publicado en esta revista (ej.: publicar en repositorio institucional o como capítulo de libro), con reconocimiento de autoría y publicación inicial en esta revista.
A los autores se les permite, y son estimulados, a publicar y distribuir su trabajo online (ej.: en repositorios institucionales o en su página personal) en cualquier punto antes o durante el proceso editorial, ya que esto puede generar alteraciones productivas, bien como aumentar el impacto y la citación del trabajo publicado (disponible en El Efecto del Acceso Libre en el link http://opcit.eprints.org/oacitation-biblio.html).


