Production of amylases by wild yeasts in response to different culture conditions
DOI:
https://doi.org/10.20873/jbb.uft.cemaf.v8n3.coelhoKeywords:
central composite rotatable design, enzyme, Plackett & Burman design, response surface methodology, starchAbstract
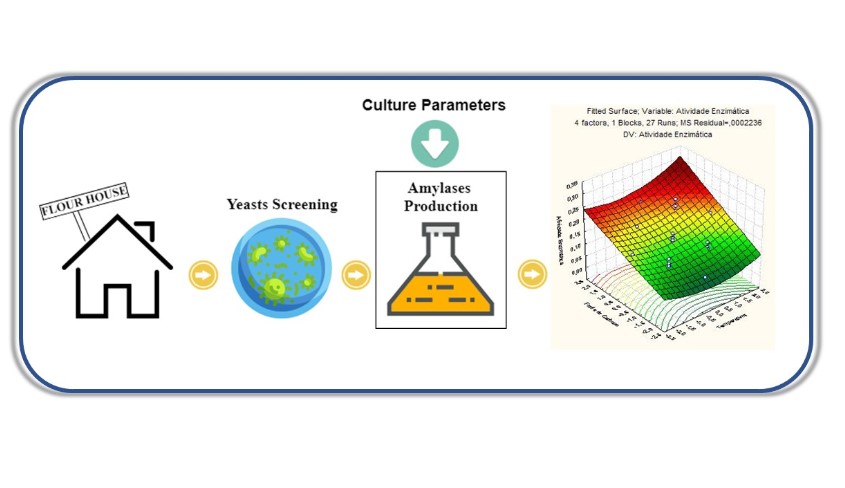
Ethanol can be produced from several raw starch species, as well as from their wastes. Hence, in view of several raw material for ethanol production it is necessary to study and select wild microorganism strains, which demonstrate the ability to hydrolyze starch into fermentable sugars. Thus, the aim of this study was investigated, under submerged culture, the statistical effect of culture parameters on the amylases activity produced by wild yeasts, isolated from puba flour production. Plackett e Burman design (PB) was used to screen and evaluate the influence of temperature, initial pH, inoculum, starch, peptone and yeast extract concentrations on the enzyme activity. By PB results was observed that temperature, inoculum, and starch concentration showed significant influence (p < 0.10) over process. Central Composite Rotatable Design (CCRD) showed that amylase activity is influenced by linear effect of temperature and soluble starch concentration (p < 0.05). Finally, response surface methodology revealed that the optimum amylase activity could be obtained with increasing of starch concentration and temperature. Thus, the results obtained in the current study suggest that the amylases produced by the PB-34 strain have potential for future studies related with the production of ethanol from starchy materials.
References
Abdel-Rahman ES, Abdel-Raheam HED, Ragab W. Optimiza-tion of α-amylase and amyloglucosidase productions from some amylolytic yeast strains. Pakistan Journal of Food Sci-ences, v.26, n.4, p.187-197, 2016.
Amid M, Manap A, Yazid M, Zohdi N. Optimization of pro-cessing parameters for extraction of amylase enzyme from dragon (Hylocereuspolyrhizus) peel using response surface methodology. The Scientific World Journal, v.2014, 2014. https:// doi.org/10.1155/2014/640949
Andualem B, Gessesse A. Isolation and identification of amyl-ase producing yeasts in ‘Tella’ (Ethiopian local beer) and their amylase contribution for ‘Tella’ production. The Jour-nal of Microbiology, Biotechnology and Food Sciences, v.3, n.1, p.30, 2013.
Casey GP, Magnus CA, Ingledew WH. High-gravity brew-ing: effects of nutrition on yeast composition, fermentative ability, and alcohol production. Applied and Environmental Microbiology, v.48, n.3, p.639-646, 1984.
Chupron J, Bovornreungroj P, Ahmad M, Kantachote D, Enomoto T. Statistical optimization for the improved pro-duction of an extracellular alkaline nuclease by halotolerant Allobacillushalotolerans MSP69: Scale-up approach and its potential as flavor enhancer of fish sauce. Biocatalysis and Agricultural Biotechnology, v.8, p.236-247, 2016. https://doi.org/10.1016/j.bcab.2016.09.011
Costa STC, Abreu-Lima TL, Carreiro SC. Atividade amilolíti-ca de leveduras isoladas de batata-doce (Ipomoea batatas (L.) Lam). Revista Biociências, v.17, n.2, 2011.
Divya K, Padma PN. Screening of diverse organic and inor-ganic nitrogen sources for cold-active polygalacturonase and amylase production by Geotrichum sp using Plackett-Burman design. International Journal for Technological Re-search in Engineering, v.3, p. 875-878, 2016.
Galdino AS, Silva RN, Lottermann MT, Álvares ACM, Mo-raes LMP, Torres FAG, Freitas SM, Ulhoa CJ. Biochemical and structural characterization of amyl 1: an alpha-amylase from Cryptococcus flavus expressed in Saccharomyces cerevisiae. Enzyme Research, v.2011, 2011. https://doi.org/10.4061/2011/157294
Guedes EHS, de Almeida DT, Abreu-Lima TL, Carreiro SC. Atividade celulolítica de leveduras isoladas de frutos de palmeiras. Boletim do Centro de Pesquisa de Processamento de Alimentos, v.36, n.1, 2019. http://dx.doi.org/10.5380/bceppa.v36i1.57644
Hankin L, Anagnostakis SL. Solid medium containing car-boxymethylcellulose to detect Cx cellulase activity of micro-organisms. Microbiology, v.98, n.1, p.109-115, 1977. https://doi.org/10.1099/00221287-98-1-109
Hashemi M, Shojaosadati SA, Razavi SH, Mousavi SM. Different catalytic behavior of a-amylase in response to the nitrogen substance used in the production phase. Journal of Industrial and Engineering Chemistry, v.21, p.772-778, 2015. https://doi.org/10.1016/j.jiec.2014.04.011
Lee JM. Biochemical Engineering. Englewood Cliffs: Prentice, NJ: Prentice Hall. 321p. 1992.
Lei H, Zhao H, Yu Z, Zhao M. Effects of wort gravity and nitrogen level on fermentation performance of brewer’s yeast and formation of flavor volatiles. Applied Biochemis-try and Biotechnology, v.166, n.6, p.1562-1574, 2012. https://doi.org/10.1007/s12010-012-9560-8.
Miller GL. Use of dinitrosalicycle acid reagent for determina-tion of reducing sugars. Analytical Chemistry, v.31, n.3, p.226-248, 1959. https:// doi.org/10.1021/ac60147a030
Montegomery DC. Design and analysis of experiments. USA: John Wiley e Sons. 287p. 2013.
Oliveira APA, Silvestre MA, Alves-Prado HF, Rodrigues A, Paz MF, Fonseca GG, Leite RSR. Bioprospecting of yeasts for amylase production in solid state fermentation and evalu-ation of the catalytic properties of enzymatic extracts. Afri-can Journal of Biotechnology, v.14, n.14, p.1215-1223, 2015. https:// doi.org/10.5897/AJB2014.14062
Paludo GB, de Abreu-Lima TL, Carreiro SC. Potencial en-zimático de leveduras isoladas de folhas em decomposição. Acta Tecnológica, v.13, n.2, p.65-77, 2019. http://dx.doi.org/10.35818/acta.v13i2.666.
Pothiraj C, Arun A, Eyinis M. Simultaneous saccharification and fermentation of cassava waste for ethanol production. Biofuel Research Journal, v.2, n.1, p.196-202, 2015. https://doi.org/10.18331/BRJ2015.2.1.5
R Development Core Team (2011). R: A language and envi-ronment for statistical computing. R Foundation for Statisti-cal Computing, Vienna, Austria. ISBN 3-900051-07-0.
Ray RR, Dutta W, Sur D, Kundu A. Optimization of fermenta-tion parameters for the production of extracellular endoglu-canase, β–glucosidase and endoxylanase by a chromium re-sistant strain of Trichoderma pseudokoningii. Journal of Microbiology, Biotechnology and Food Sciences, v.3, n.1, p.54-58, 2013.
Scriban R. Biotecnologia.São Paulo: Manole. 488p. 1985.
Swetha S, Varma A, Padmavathi T. Statistical evaluation of the medium components for the production of high biomass, α-amylase and protease enzymes by Piriformosporaindica using Plackett–Burman experimental design. 3 Biotech, v.4, n.4, p.439-445, 2014. https://doi.org/10.1007/s13205-013-0168-7
Viktor MJ, Rose SH, Van Zyl WH, Viljoen-Bloom M. Raw starch conversion by Saccharomyces cerevisiae expressing Aspergillus tubingensis amylases. Biotechnology for Biofu-els, v.6, n.1, p.167-175, 2013. https://doi.org/10.1186/1754-6834-6-167.
Wang F, Jian Y, Guo W, Niu K, Zhang R, Hou S, Wang M, Yi Y, Zhu C, Jia C, Fang, X. An environmentally friendly and productive process for bioethanol production from pota-to waste. Biotechnology for Biofuels, v.9, n.1, p.50, 2016. https://doi.org/10.1186/s13068-016-0464-7
Xu Q-S, Yan Y-S, Feng J-X. Efficient hydrolysis of raw starch and ethanol fermentation: a novel raw starch-digesting glucoamylase from Penicillium oxalicum. Biotechnology for Biofuels, v.9, n.1, p.216-233, 2016. https://doi.org/10.1186/s13068-016-0636-5
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 - Journal of Biotechnology and Biodiversity

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License (CC BY 4.0 at http://creativecommons.org/licenses/by/4.0/) that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
Authors are permitted and encouraged to post their work online (e.g. in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (Available at The Effect of Open Access, at http://opcit.eprints.org/oacitation-biblio.html).


