White fungal biotechnology: prospecting and identifying anemophilic fungi for possible applications in industrial processes.
DOI:
https://doi.org/10.20873/jbb.uft.cemaf.v13n3.20417Resumen
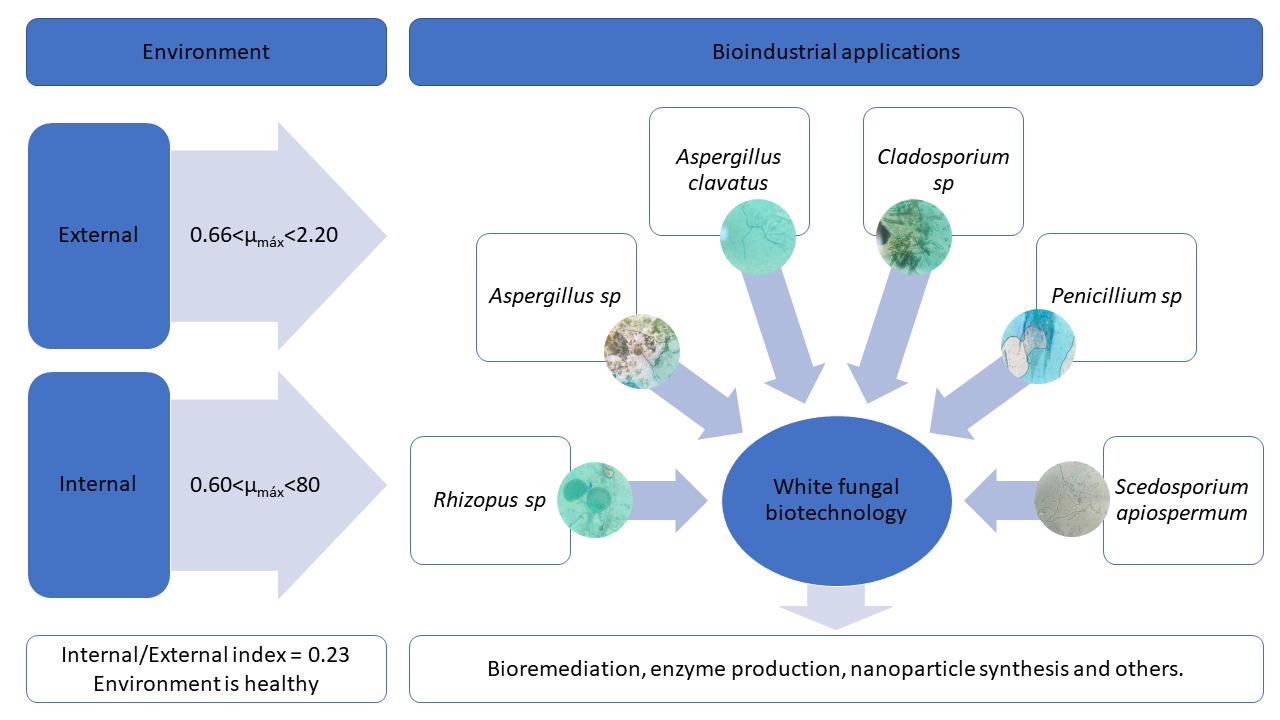
White biotechnology focuses on industrial processes, being an interesting field for the application of fungi. In this sense, microorganisms were prospected both in the external and internal environment of a university. The passive sedimentation technique was conducted, using sterile 50 mm Petri dishes. The collections were in 10 environments (5 open and 5 closed), being carried out in replicate. The Tukey statistical test was used.. In open locations, a greater quantity of microorganisms was quantified, in addition to having a more vigorous maximum specific growth rate. Through the ratio between microorganisms in the Internal/External environments of 0.23, it was found that the study environment is healthy, despite having a microbial presence. Rhizopus sp, Aspergillus sp, Cladosporium sp, Penicillium sp and Scedosporium sp were identified, which could be applied in white biotechnological processes such as bioremediation, enzyme production, nanoparticle synthesis and others. In this way, microorganisms with real potential for industrial applications were prospected.
Citas
Abrego, Nerea; Crosier, Brittni; Somervuo, Panu; Ivanova, Natalia; Abrahamyan, Arusyak; Abdi, Amir; Hämäläinen, Karoliina; Junninen6, Kaisa; Maunula, Minna; Purhonen, Jenna; Ovaskainen, O. Fungal communities decline with ur-banization—more in air than in soil. The ISME Journal, v. 14, n.11, p. 2806-2815, 2020. https://doi.org/10.1038/s41396-020-0732-1
Acevedo, Y. S. M.; Mancera, L. T. M.; Moreno-Piraján, J. C.; Flórez, M. V. Regeneration of activated carbon by applying the phenolic degrading fungus Scedosporium apiosper-mum. Journal of Environmental Chemical Engineering, v. 8, e103691, 2020. https://doi.org/10.1016/j.jece.2020.103691
Agertt, M.; Arabidian, L.; Cademartori, C.; Beneduzi, A. Ocorrência de fungos filamentosos no ambiente de uma se-ção de coleções especiais. SaBios-Revista de Saúde e Biolo-gia, v. 17, p. 1-10, 2022. https://doi.org/10.54372/sb.2022.v17.3348
Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M. I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium ox-ysporum. Colloids and surfaces B: Biointerfaces, v.28, p. 313-318, 2003.
https://doi.org/10.1016/S0927-7765(02)00174-1
Asemoloye, Michael Dare; Tosi, Solveig; Daccò, Chiara; Wang, Xiao; Xu, Shihan; Marchisio, Mario Andrea; Gao, Wenyuan; Jonathan, Segun Gbolagade; Pecoraro, Lorenzo. Hydrocarbon degradation and enzyme activities of Aspergil-lus oryzae and Mucor irregularis isolated from nigerian crude oil-polluted sites. Microorganisms, v.8, n. 12, 2020. https://doi.org/10.3390/microorganisms8121912
Atakpa, E. O.; Zhou, H.; Jiang, L.; Zhang, D.; Li, Y.; Zhang, W.; Zhang, C. Co-culture of Acinetobacter sp. and Scedosporium sp. immobilized beads for optimized biosur-factant production and degradation of crude oil. Environmental Pollution, v. 335, p.122365, 2023. https://doi.org/10.1016/j.envpol.2023.122365
Benabda, O.; M’hir, S.; Kasmi, M.; Mnif, W.; Hamdi, M. Optimization of protease and amylase production by Rhizo-pus oryzae cultivated on bread waste using solid-state fer-mentation. Journal of Chemistry, v. 2019, p.1-9, 2019. https://doi.org/10.1155/2019/3738181
Bernardi, E.; Da Costa, E. L. G.; Do Nascimento, J. S. Fungos anemófilos e suas relações com fatores abióticos, na praia do Laranjal, Pelotas, RS. Revista de Biologia e Ciências da Ter-ra, v. 7, n. 2, 2007. https://seer.ufrgs.br/index.php/rbrasbioci/article/view/114721
Brasil. Agência Nacional De Vigilância Sanitária. Resolução Nº 9, De 16 De Janeiro De 2003. Orientação Técnica elabo-rada por Grupo Técnico Assessor, sobre Padrões Referenci-ais de Qualidade do Ar Interior, em ambientes climatizados artificialmente de uso público e coletivo. Diário Oficial da União, 20 de janeiro de 2003.
Caixeta, D. S.; Silva, T. A.; Santana, F. M. F.; Almeida, W. T. P. Monitoramento da Qualidade do Ar Interior de uma Es-cola da Rede Pública Localizada no Município de Cuiabá-MT. E&S Engineering and Science, v. 5, n. 1, p. 20-28, 2016.
https://doi.org/10.18607/ES201653207
Campos, F. M.; Golin, R.; Caixeta, F. C.; Sanches, L.; Cai-xeta, D. S. Avaliação quanti-qualitativa do ar interior de uma biblioteca pública do município de Cuiabá-MT. E&S Engi-neering and Science, v. 6, n. 1, p. 95-105, 2017.
https://doi.org/10.18607/ES201764756
Chidichima, L.P.S.; Nozaki, M.H.; Hendges, C.; Gaias, W.L. Crescimento e etiologia do agente causal da antracnose em morangueiro. Acta Iguazu, v. 7, n. 4, p. 24-34, 2018. https://doi.org/10.48075/actaiguaz.v7i4.17459
Chinchilla, P. C.; Larua, I. A. de. Las bioindustrias en el en-torno de la bioeconomía necessidades, oportunidades y be-neficios. Mediterráneo Económico, Almeria, v. 31, p. 203-217, 2018. https://dialnet.unirioja.es/servlet/articulo?codigo=6648777.
COELHO, E.; REIS, T. A.; COTRIM, M.; RIZZUTTO, M.; & CORRÊA, B. Bioremediation of water contaminated with uranium using Penicillium piscarium. Biotechnology Pro-gress, v. 36, n. 5, 2020. https://doi.org/10.1002/btpr.3032
Costa, A. C. A. D.; Lino, L. A. D. S.; Hannesch, O. Micro-organismos em áreas de guarda de acervos científicos do Museu de Astronomia e Ciências Afins: adequação aos pa-drões nacionais de qualidade de ar. Boletim Eletrônico da ABRACOR, v. 5, p. 1-12, 2011. Available at: https://www.researchgate.net/publication/268220905
Cowan, A. R.; Costanzo, C. M.; Benham, R.; Loveridge, E. J.; Moody, S. C. Fungal bioremediation of polyethylene: Chal-lenges and perspectives. Journal of Applied Microbiolo-gy, v. 132, n. 1, p. 78-89, 2022. https://doi.org/10.1111/jam.15203
Cunha, V. A. M. G.; Oliveira, D. A. B.; Bombana, C. C.; Pavanelli, M. F.; Parussolo, L. Quantificação de Fungos e Bactérias Para Avaliação do Ar Interno de uma Empresa da Região Centro-Oeste do Paraná. Saúde e Pesquisa, v. 6 n. 3, 2013. Available at: https://periodicos.unicesumar.edu.br/index.php/saudpesq/article/view/2949
Doran, P. M. Bioprocess engineering principles. Elsevier, 2º edição, 2012. 919p.
Dsouza, G. C.; Sheriff, R. S.; Ullanat, V.; Shrikrishna, A.; Joshi, A. V.; Hiremath, L.; Entoori, K. Fungal biodegrada-tion of low-density polyethylene using consortium of As-pergillus species under controlled conditions. Heliyon, v. 7, n.5, 2021.
https://doi.org/10.1016/j.heliyon.2021.e07008
El-Hawary, S. S.; Moawad, A. S.; Bahr, H. S.; Abdelmohsen, U. R.; Mohammed, R. Natural product diversity from the endophytic fungi of the genus Aspergillus. RSC advances, v. 10, n. 37, p. 22058-22079, 2020. https://doi.org/10.1039/D0RA04290K
Elizei, V. G.; Chalfoun, S. M.; Botelho, D. M. S.; Rebelles, P. P. R.. Imobilização de fungos filamentosos com potencial para uso agroindustrial. Arquivos do Instituto Biológico, v. 81, n. 2, p. 165-172, 2014. https://doi.org/10.1590/1808-1657001032012
Espinoza-Sanchez, M. A.; Arevalo-Nino, K.; Quintero-Zapata, I.; Castro-Gonzalez, I.; Almaguer-Cantu. Cr (VI) adsorption from aqueous solution by fungal bioremediation based using Rhizopus sp. Journal of Environmental Management, v. 251, p. 109595, 2019. https://doi.org/10.1016/j.jenvman.2019.109595
Es-Haghi, A.; Taghavizadeh Yazdi, M. E.; Sharifalhoseini, M.; Baghani, M.; Yousefi, E.; Rahdar, A.; Baino, F. Appli-cation of response surface methodology for optimizing the therapeutic activity of ZnO nanoparticles biosynthesized from Aspergillus niger. Biomimetics, v. 6, n. 2, 2021. https://doi.org/10.3390/biomimetics6020034
Ganesan, V.; Hariram, M.; Vivekanandhan, S.; Muthuramku-mar, S. Periconium sp.(endophytic fungi) extract mediated sol-gel synthesis of ZnO nanoparticles for antimicrobial and antioxidant applications. Materials Science in Semiconductor Processing, v. 105, p. 104739 2020. https://doi.org/10.1016/j.mssp.2019.104739
Ge, J.; Jiang, X.; Liu, W.; Wang, Y.; Huang, H.; Bai, Y.; Su, X.; Yao, B.; Luo, H. Characterization, stability improve-ment, and bread baking applications of a novel cold-adapted glucose oxidase from Cladosporium neopsychrotolerans SL16. Food Chemistry, v. 310, p. 125970, 2020. https://doi.org/10.1016/j.foodchem.2019.125970
Gołofit-Szymczak, M.; Stobnicka-Kupiec, A.; Górny, R. L. Impact of air-conditioning system disinfection on microbial contamination of passenger cars. Air Quality, Atmosphere & Health, v. 12, p. 1127-1135, 2019. https://doi.org/10.1007/s11869-018-0614-0
Hamed, M.; Osman, A. A.; Ateş, M. Statistical optimization of L-asparaginase production by Cladosporium tenuissi-mum. Egyptian Pharmaceutical Journal, v. 20, n. 1, p.51-58, 2021.
https://doi.org/10.4103/epj.epj_47_20
Hasanin, M. S.; Hashem, A. H.; Abd El-Sayed, E. S.; & El-Saied, H. Green ecofriendly bio-deinking of mixed office waste paper using various enzymes from Rhizopus micro-sporus AH3: efficiency and characteristics. Cellulose, v. 27, p. 4443-4453, 2020.
https://doi.org/10.1007/s10570-020-03071-3
Heux, S.; Meynial-Salles, I.; O'donohue, M. J.; Dumon, C. White biotechnology: State of the art strategies for the de-velopment of biocatalysts for biorefining. Biotechnology Advances, Toulouse, v. 33, n. 8, p. 1653-160, 2015. https://doi.org/10.1016/j.biotechadv.2015.08.004
Hyde, K. D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A. G. T.; ... & Stadler, M. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Di-versity, v. 97, p. 1-136, 2019. https://doi.org/10.1007/s13225-019-00430-9
Juárez-Hernández, J.; Castillo-Hernández, D.; Pérez-Parada, C.; Nava-Galicia, S.; Cuervo-Parra, J. A.; Surian-Cruz, E.; Godínez, G. D.; Sánchez, C.; Bibbins-Martínez, M. Isola-tion of fungi from a textile industry effluent and the screen-ing of their potential to degrade industrial dyes. Journal of Fungi, v. 7, n. 10, 2021. https://doi.org/10.3390/jof7100805
Kumaravel, V.; Bankole, P. O.; Jooju, B.; Sadasivam, S. K. Degradation and detoxification of reactive yellow dyes by Scedosporium apiospermum: a mycoremedial ap-proach. Archives of Microbiology, v. 204, n. 6, 2022. https://doi.org/10.1007/s00203-022-02947-1
Kumar, A. Aspergillus nidulans: a potential resource of the production of the native and heterologous enzymes for in-dustrial applications. International Journal of Microbiology, v. 8894215, 2020. https://doi.org/10.1155/2020/8894215
Lacaz, C.S.; Porto, E.; Heins-Vaccari, E.M.; Melo, N. T. (1998). Guia para identificação: fungos, actinomicetos,algas de interesse médico. São Paulo: Sarvier, 1998. 445 p.
Liu, Z.; Deng, Y.; Ma, S.; He, B. J.; & Cao, G. Dust accumu-lated fungi in air-conditioning system: Findings based on field and laboratory experiments. In Building simulation v. 14, p. 793-811, 2021.
https://doi.org/10.1007/s12273-020-0693-3
Liu, J.; Zeng, Q.; Lei, H.; Xin, K.; Xu, A.; Wei, R.; Li, D.; Zhou, J.; Dong, W.; Jiang, M. Biodegradation of polyester polyurethane by Cladosporium sp. P7: Evaluating its degra-dation capacity and metabolic pathways. Journal of Hazard-ous Materials, v. 448, 130776, 2023. https://doi.org/10.1016/j.jhazmat.2023.130776
López-Fernández, J.; Benaiges, M. D.; Valero, F. Rhizopus oryzae lipase, a promising industrial enzyme: Biochemical characteristics, production and biocatalytic applica-tions. Catalysts, v. 10, n. 11, p. 1277, 2020. https://doi.org/10.3390/catal10111277
Lotfy, W. A.; Alkersh, B. M.; Sabry, S. A.; Ghozlan, H. A. Biosynthesis of silver nanoparticles by Aspergillus terreus: characterization, optimization, and biological activi-ties. Frontiers in bioengineering and biotechnology, v. 9, 2021. https://doi.org/10.3389/fbioe.2021.633468
Mani, V. M.; Kalaivani, S.; Sabarathinam, S.; Vasuki, M.; Soundari, A. J. P. G.; Das, M. A.; Elfasakhany, A.; Puga-zhendhi, A. Copper oxide nanoparticles synthesized from an endophytic fungus Aspergillus terreus: Bioactivity and anti-cancer evaluations. Environmental Research, v. 201, p. 111502, 2021.
https://doi.org/10.1016/j.envres.2021.111502
Melnichuk, N.; Braia, M. J.; Anselmi, P. A.; Meini, M. R.; & Romanini, D. Valorization of two agroindustrial wastes to produce alpha-amylase enzyme from Aspergillus oryzae by solid-state fermentation. Waste Management, v. 106, p. 155-161, 2020.
https://doi.org/10.1016/j.wasman.2020.03.025
Méndez-Líter, J. A.; De Eugenio, L. I.; Nieto-Domínguez, M.; Prieto, A.; Martínez, M. J. Hemicellulases from Penicillium and Talaromyces for lignocellulosic biomass valorization: A review. Bioresource Technology, v. 324, p. 124623, 2021. https://doi.org/10.1016/j.biortech.2020.124623
Meyer, V.; Basenko, E. Y.; Benz, J. P.; Braus, G. H.; Cad-dick, M. X.; Csukai, M.; ... & Wösten, H. A. Growing a circular economy with fungal biotechnology: a white paper. Fungal biology and biotechnology, v. 7, n. 1, p. 1-23, 2020.
https://doi.org/10.1186/s40694-020-00095-z
Miranda, E. D. S. C.; Costa, M. E. R. L.; Nascimento, J. C. N.; Atayde, H. M. Desempenho de diferentes meios de cul-tura na quantificação da micobiota contaminante de piracuí no período chuvoso. Open Science Research X, v. 10, n. 1, p. 91-99, 2023.
https://doi.org/10.37885/230111748
Moghaddam AB, Moniri M, Azizi S, Rahim RA, Ariff AB, Saad WZ, Namvar F, Navaderi M, Mohamad R. Biosynthe-sis of ZnO Nanoparticles by a New Pichia kudriavzevii Yeast Strain and Evaluation of Their Antimicrobial and An-tioxidant Activities. Molecules, v. 22, n. 872, p. 1-18, 2017.
https://doi.org/10.3390/molecules22060872
Morales-Oyervides, L.; Ruiz-Sánchez, J. P.; Oliveira, J. C.; Sousa-Gallagher, M. J.; Méndez-Zavala, A.; Giuffrida, D.; ... & Montañez, J. Biotechnological approaches for the pro-duction of natural colorants by Talaromyces/Penicillium: A review. Biotechnology Advances, v. 43, p. 107601, 2020. https://doi.org/10.1016/j.biotechadv.2020.107601
Mushimiyimana, I.; Umuhozariho, M. G.; & Maniraho, L. A statistical strategy for the production of cellulase, xylanase and α-amylase by Cladosporium cladosporioides. Fungal Territory, v. 2, n. 2, p. 16-21, 2019. https://doi.org/10.36547/ft.2019.2.2.16-21
Nageen, Y.; Asemoloye, M.D.; Põlme, S. et al. Analysis of culturable airborne fungi in outdoor environments in Tianjin, China. BMC Microbiol v. 21, n. 134, 2021. https://doi.org/10.1186/s12866-021-02205-2
Neto, J. O.; Silva, T. M. R. M.; & Travareli, M. M. Avaliação Microbiologica Da Qualidade Do Ar Em Ambientes Clima-tizados Da Unicamp Através De Contagem De Fun-gos. Revista Ciências do Ambiente On-Line, v. 9, n.1, 2013. Available at: https://sistemas.ib.unicamp.br/be310/nova/index.php/be310/article/view/348/274
Njoku, K. L.; Akinyede, O. R.; Obidi, O. F. Microbial remedi-ation of heavy metals contaminated media by Bacillus mega-terium and Rhizopus stolonifer. Scientific African, v, 10, e00545, 2020. https://doi.org/10.1016/j.sciaf.2020.e00545
Nobre, C.; Do Nascimento, A. K. C.; Silva, S. P.; Coelho, E.; Coimbra, M. A.; Cavalcanti, M. T. H.; Teixeira, J. A.; Por-to, A. L. F. Process development for the production of prebiotic fructo-oligosaccharides by Penicillium citre-onigrum. Bioresource technology, v. 282, p. 464-474, 2019.
https://doi.org/10.1016/j.biortech.2019.03.053
Oliveira, M.; Abreu, I.; Ribeiro, H.; Delgado, L. Esporos fúngicos na atmosfera da cidade do Porto e suas implicações alergológicas. Revista Portuguesa de Imunoalergologia, v. 15, n. 1, p. 61-85, 2007. Available at: https://www.spaic.pt/client_files/rpia_artigos/esporos-fungicos-na-atmosfera-da-cidade-do-porto-e-suas-implicacoes-alergologicas.pdf
Pan, F.; Yang, N.; Zhu, X.; Yu, C.; Jiang, M.; Jiang, Y.; Liu, S.; Wu, W.; Liu, Y. Discovery of a natural hybrid polyke-tide produced by endophytic cladosporium sphaerospermum for biocontrol of phytopathogenic fungus Botrytis ciner-ea. Journal of Agricultural and Food Chemistry, v. 71, n. 32, p. 12190-12202, 2023. http://doi.org/10.1021/acs.jafc.3c02408
Pereira, A. L.; Pita, J. R. Alexander Fleming (1881-1955): da descoberta da penicilina (1982) ao prémio Nobel (1945). História: revista da Faculdade de Letras da Univer-sidade do Porto, v. 6, 2018.
Quintella, C. M.; Mata, A. M.; Lima, L. C. Overview of bio-remediation with technology assessment and emphasis on fungal bioremediation of oil contaminated soils. Journal of environmental management, v. 241, p. 156-166, 2019. https://doi.org/10.1016/j.jenvman.2019.04.019
Răut, I.; Călin, M.; Capră, L.; Gurban, A. M.; Doni, M.; Radu, N.; Jecu, L. Cladosporium sp. isolate as fungal plant growth promoting agent. Agronomy, v. 11, n. 2, 2021. https://doi.org/10.3390/agronomy11020392
Raven, S.; Francis, A.; Srivastava, C.; Kezo, S.; Tiwari, A. Fungal Biology Recent Advancement in White Biotechnolo-gy Through Fungi Volume 2: Perspective for Value-Added Products and Environments, v. 2 p. 385-405, 2019.
Sales, E.; Sales, E. M. L.; Dias, L. F.; De Carvalho Costa, F. E.; Loyola, A. B. A. T. Micota no ar da unidade de terapia intensiva e centro cirúrgico de um hospital universitá-rio. Bioikos–Título não-corrente, v. 25 n. 2, 2011.Available at: https://periodicos.puc-campinas.edu.br/bioikos/article/view/550
Saini, S.; Kumar, A.; Singhal, B.; Kuhad, R. C.; & Sharma, K. K. Fungal oxidoreductases and CAZymes effectively de-grade lignocellulosic component of switchgrass for bioetha-nol production. Fuel, v. 328, p. 125341, 2022. https://doi.org/10.1016/j.fuel.2022.125341
Šantl-Temkiv, T.; Amato, P.; Casamayor, O. E.; Lee, P. K. H.; Pointing, S. B. Microbial ecology of the atmosphere. Fems Microbiology Reviews, v. 46, n. 4, p. 1-18, 2022. https://doi.org/10.1093/femsre/fuac009
Saye, Lm, Navaratna, Ta, Chong, Jp, O'malley, Ma, Theodor-ou, Mk, & Reilly, M. The Anaerobic Fungi: Challenges and Opportunities for Industrial Lignocellulosic Biofuel Produc-tion Microorganisms, v, 9, n. 4, 2021. https://doi.org/10.3390/microorganisms9040694
Seddiek, H. A.; Shetaia, Y. M.; Mahamound, K. F.; El-Aassy, I. E.; Hussien, S. S. Bioleaching of Egyptian fly ash using cladosporium cladosporioides. Ann. Biol, v. 37, p.18-22, 2021.
https://www.cabidigitallibrary.org/doi/pdf/10.5555/20210261803
Siebra, C. M.; De Freitas, J. V. P.; Pantoja, L. D. M.; Paixão, G. C. Qualidade do ar em ambientes internos e externos de um cemitério do município de Fortaleza, Ceará. Ambiente: Gestão e Desenvolvimento. 2021. https://doi.org/10.24979/ambiente.v1i1.939
Solairaj, D.; Legrand, N. N. G.; Yang, Q.; Liu, J.; Zhang, H. Microclimatic parameters affect Cladosporium rot develop-ment and berry quality in table grapes. Horticultural Plant Journal, v. 8, n. 2, p. 171-183, 2022. https://doi.org/10.1016/j.hpj.2021.07.002
Spilak MP, Madsen AM, Knudsen SM, Kolarik B, Hansen EW, Frederiksen M, Gunnarsen L. 2015. Impact of dwell-ing characteristics on concentrations of bacteria, fungi, endo-toxin and total inflammatory potential in settled dust. Build-ing and Environment 93:64-71. https://doi.org/10.1016/j.buildenv.2015.03.031
Suciatmih, S. Combination of culture and dyeing conditions on cloth color dyed with mixed fungi dyes. Berkala Penelitian Hayati, v. 25, n. 2, p. 18-26, 2020. http://dx.doi.org/10.23869/bphjbr.25.2.20202
Tortora, Gerard J.; Case, Christine L.; Funke, Berdell R. Microbiologia-12ª Edição. Artmed Editora, 2016. 964p.
Troiano, D.; Orsat, V.; Dumont, M. J. Status of filamentous fungi in integrated biorefineries. Renewable and Sustainable Energy Reviews, Belfast, v. 117, p.109472, 2020. https://doi.org/10.1016/j.rser.2019.109472
Unifei. Universidade Federal de Itajubá. 2023. Available at: https://unifei.edu.br/institucional/mapa-do-campus-itajuba/.
Valério, R. B. R.; Cavalcante, A. L. G.; Mota, G. F.; De Sou-sa, I. G.; Souza, J. E. S.; Cavalcante, F. T. T.; Moreira, K. S.; Falcão, I. R. A.; Neto, F. S.; Dos Santos, J. C. S. Un-derstanding the biocatalytic potential of lipase from Rhizo-pus chinensis. Biointerface Res. Appl. Chem, v. 12, p. 4230-4260, 2022. https://doi.org/10.33263/BRIAC123.42304260
Velmurugan, P.; Kim, M. J.; Park, J. S.; Karthikeyan, K.; Lakshmanaperumalsamy, P.; Lee, K. J.; Park, Y. J.; Oh, B. T. Dyeing of cotton yarn with five water soluble fungal pigments obtained from five fungi. Fibers and Polymers, v. 11, p. 598-605, 2010.
https://doi.org/10.1007/s12221-010-0598-5
Venil, C. K.; Velmurugan, P.; Dufossé, L.; Renuka Devi, P.; Veera Ravi, A. Fungal pigments: Potential coloring com-pounds for wide ranging applications in textile dye-ing. Journal of fungi, v. 6, n. 2, 2020. https://doi.org/10.3390/jof6020068
Wang, D.; Xue, B.; Wang, L.; Zhang, Y.; Liu, L.; Zhou, Y. (2021). Fungus-mediated green synthesis of nano-silver us-ing Aspergillus sydowii and its antifungal/antiproliferative activities. Scientific reports, v. 11, n. 1, p.10356, 2021. https://doi.org/10.1038/s41598-021-89854-5
Wang, F.; Xu, L.; Zhao, L.; Ding, Z.; Ma, H.; Terry, N. Fun-gal laccase production from lignocellulosic agricultural wastes by solid-state fermentation: a re-view. Microorganisms, v. 7, n. 12, 2019.
https://doi.org/10.3390/microorganisms7120665
Wösten, Han Ab. Filamentous fungi for the production of enzymes, chemicals and materials. Current opinion in bio-technology, v. 59, p. 65-70, 2019. https://doi.org/10.1016/j.copbio.2019.02.010
Wood, D. L.; Tauc, J. S. Weak absorption tails in amorphous semiconductors. Physical Review B, v. 5, n. 8, p. 3144, 1972.
https://doi.org/10.1103/PhysRevB.5.3144
Yang, M.; Xu, W.; Li, J.; Zhou, Z.; Lu, Y. A modified version of BRDF model based on Kubelka-Munk theory for coating materials. Optik, v. 193, p. 162982, 2019. https://doi.org/10.1016/j.ijleo.2019.162982
Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Bi, Q.; Zhao, Y. Biodegradation of polyethylene microplastic parti-cles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Science of the Total Environ-ment, v. 704, p.135931, 2020. https://doi.org/10.1016/j.scitotenv.2019.135931
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2025 Mônica Suelen dos Santos Ribeiro, Camila Silva Pereira, Ana Paula Moni Silva, Paulo Sérgio Marques, Márcio Daniel Nicodemos Ramos

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Copyright (c) 2024 - Journal of Biotechnology and Biodiversity

Esta obra está bajo una Licencia Creative Commons Atribución 4.0 Internacional.
Los autores que publican en esta revista aceptan los siguientes términos:
Los autores mantienen los derechos autorales y conceden a la revista el derecho de primera publicación, con el trabajo simultáneamente licenciado bajo la LicenciaCreative Commons Attribution (CC BY 4.0 en el link http://creativecommons.org/licenses/by/4.0/) que permite compartir el trabajo con reconocimiento de la autoría y publicación inicial en esta revista.
Los autores tienen autorización para asumir contratos adicionales separadamente, para distribución no exclusiva de la versión del trabajo publicado en esta revista (ej.: publicar en repositorio institucional o como capítulo de libro), con reconocimiento de autoría y publicación inicial en esta revista.
A los autores se les permite, y son estimulados, a publicar y distribuir su trabajo online (ej.: en repositorios institucionales o en su página personal) en cualquier punto antes o durante el proceso editorial, ya que esto puede generar alteraciones productivas, bien como aumentar el impacto y la citación del trabajo publicado (disponible en El Efecto del Acceso Libre en el link http://opcit.eprints.org/oacitation-biblio.html).


