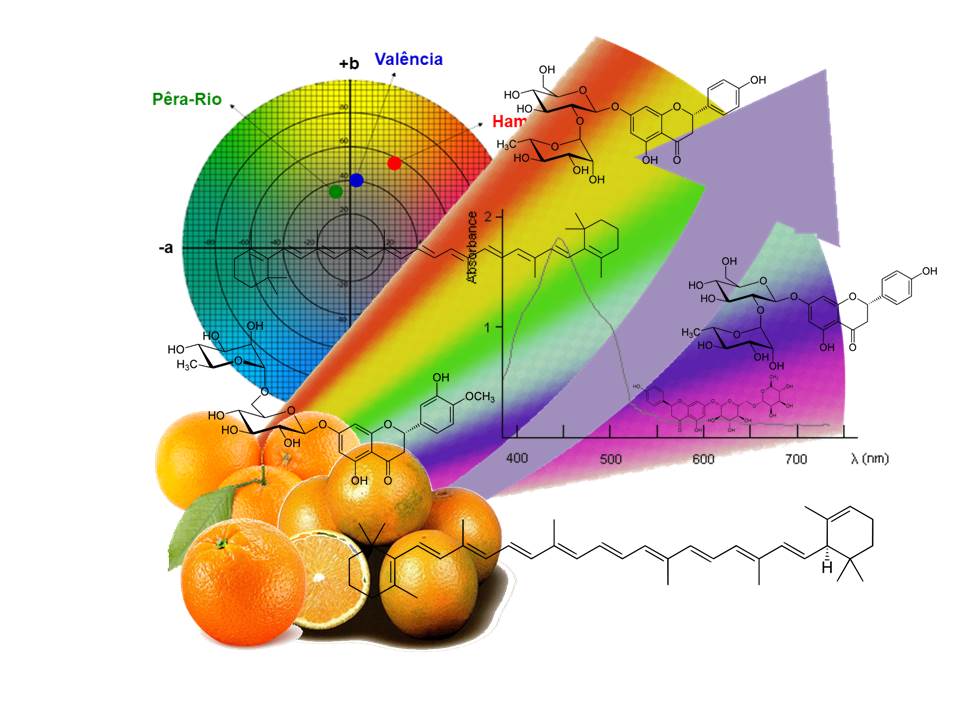
Classificação quimiotaxonômica de laranjas doces relacionadas (Citrus sinensis L.) utilizando análise de componentes principais e análise de agrupamento de cor e compostos bioativos nas cascas das laranjas
DOI:
https://doi.org/10.20873/jbb.uft.cemaf.v12n1.16214Palavras-chave:
laranja, classificação, composição química, metabolismo secundário, quimiometriaResumo
A quimiotaxonomia é a tentativa de classificar as plantas, de acordo com diferenças e semelhanças em seus conteúdos bioquímicos. Os metabólitos secundários são frequentemente específicos e restritos a plantas taxonomicamente relacionadas. Avaliamos a quimiotaxonomia de laranjas doces utilizadas na produção de suco concentrado, usando atividades antioxidantes, perfis de flavonoides e fenólicos e varredura de espectros UV-visível; e análise CIELab. A análise de cluster mostrou que a análise CIELab se mostrou mais eficaz. Outras técnicas devem ser aplicadas após extensa e criteriosa extração por solventes orgânicos. Todas as técnicas aplicadas provam distinguir os tipos de laranja. Grandes conjuntos de dados devem ser preparados, o que muitas vezes é difícil, mas o uso de PCA e HCA aumenta a interpretabilidade. Como o monitoramento da qualidade da fruta in natura é baseado na visualização humana subjetiva ou na análise do suco de laranja por razão, que não são muito precisos e confiáveis quanto aos teores químicos, busca-se técnicas mais rápidas para garantir a qualidade nas indústrias de suco de laranja.
Referências
Aguiar CL, Haddad R, Eberlin MN, Carrão-Panizzi MC, Tsai SM, Park YK. Thermal behavior of malonyl glucoside iso-flavones in soybean flour analyzed by RP-HPLC/DAD and electrospray ionization mass spectrometry. LWT-Food Sci-ence and Technology, v. 48, n. 1, p. 114–119, 2012. https://doi.org/10.1016/j.lwt.2012.02.017
Aguiar CL, Walder JM, Tsai SM, Carrão-Panizzi MC, Kitaji-ma EW. Changes in isoflavone profiles of soybean treated with gamma irradiation. International Journal of Food Sci-ences and Nutrition, v. 60, n. 5, p. 387-394, 2009.
https://doi.org/10.1080/09637480701754968
Baqueta MR, Coqueiro A, Março PH, Valderrama P. Quality control parameters in the roasted coffee industry: a proposal by using microNIR spectroscopy and multivariate calibra-tion. Food Analytical Methods, v. 13, p. 50–60, 2020.
https://doi.org/10.1007/s12161-019-01503-w
Basri H, Syarif I, Sukaridhoto S, Falah MF. Intelligent system for automatic classification of fruit defect using faster re-gion-based convolutional neural network (faster r-CNN). Jurnal Ilmiah Kursor, v. 10, p. 1, 2019.
Bayer RJ, Mabberley DJ, Morto C, Miller CH, Sharma IK, Pfeil BE, Rich S, Hitchcock R, Sykes S. A molecular phy-logeny of the orange subfamily (Rutaceae: Aurantioideae) using nine cpDNA sequences. American Journal of Botany, v. 96, n. 3, p. 668-685, 2009.
Blauer, R. Citrus: World markets and trade. FAS: USDA, 2023. Online: https://fas.usda.gov/data/citrus-world-markets-and-trade
Bento C, Gonçalves AC, Jesus F, Simões M, Silva LR. Phe-nolic compounds: Sources, properties and applications. Bio-active compounds: sources, properties and applications. 2017. p. 271-299.
Borello E, Domenici V. Determination of pigments in virgin and extra-virgin olive oils: a comparison between two near UV-Vis spectroscopic techniques. Foods, v. 8, n. 18, 2019.
https://doi.org/10.3390/foods8010018
Bouhafsoun A, Yilmaz MA, Boukeloua A, Temel H, Harche MK. Simultaneous quantification of phenolic acids and fla-vonoids in Chamaerops humilis L. using LC–ESI-MS/MS. Food Science and Technology, v. 38, n. 1, 2018. https://doi.org/10.1590/fst.19917
Chen J, Yang J, Ma L, Li J, Shahzad N, Kim CK. Structure-antioxidant activity relationship of methoxy, phenolic hy-droxyl, and carboxylic acid groups of phenolic acids. Scien-tific Reports, v. 10, n. 1, 2020a.
https://doi.org/10.1038/s41598-020-59451-z
Chen Q, Wang D, Tan C, Hu Y, Sundararajan B, Zhou Z. Profiling of flavonoid and antioxidant activity of fruit tissues from 27 Chinese local citrus cultivars. Plants, v. 9, n. 2, p. 196, 2020b.
https://doi.org/10.3390/plants9020196
Denaro M, Smeriglio A, Trombetta D. Antioxidant and anti-inflammatory activity of citrus flavanones mix and its stabil-ity after in vitro simulated digestion. Antioxidants, v. 10, n. 2, p. 140, 2021.
https://doi.org/10.3390/antiox10020140
Flores-Hidalgo M, Torres-Rivas F, Monzon-Bensojo J, Es-cobedo-Bretado M, Glossman-Mitnik D, Barraza-Jimenez D. Electronic structure of carotenoids in natural and artificial photosynthesis. InTech: Rijeka, 2017. p. 17-33.
Gómez-Mejía E, Rosales-Conrado N, León-González M, Madrid Y. Citrus peels waste as a source of value-added compounds: extraction and quantification of bioactive poly-phenols. Food Chemistry, v. 295, p. 289-299, 2019.
https://doi.org/10.1016/j.foodchem.2019.05.136
Granato D, Santos JS, Escher GB, Ferreira BL, Maggio RM. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends in Food Science & Technology, v. 72, p. 83-90, 2018.
https://doi.org/10.1016/j.tifs.2017.12.006
Granato D, Nunes DS, Barba FJ. An integrated strategy be-tween food chemistry, biology, nutrition, pharmacology, and statistics in the development of functional foods: A pro-posal. Trends in Food Science & Technology, v. 62, p. 13–22, 2017.
https://doi.org/10.1016/j.tifs.2016.12.010
Granato D, Calado VM, Jarvis B. Observations on the use of statistical methods in Food Science and Technology. Food Research International, v, 55, p. 137–149, 2014.
https://doi.org/10.1016/j.foodres.2013.10.024
Harnly JM, Bhagwat S, Lin LZ. Profiling methods for the determination of phenolic compounds in foods and dietary supplements. Analytical and Bioanalytical Chemistry, v. 389, n. 1, p. 47–61, 2007.
https://doi.org/10.1007/s00216-007-1424-7
Hegazy AE, Ibrahim MI. Antioxidant activities of orange peel extracts. World Applied Sciences Journal, v. 18, n. 5, p. 684-688, 2012.
http://doi.org/10.5829/idosi.wasj.2012.18.05.64179
Holser RA. Principal component analysis of phenolic acid spectra. International Scholarly Research Notices, v. 9, 2012.
https://doi.org/10.5402/2012/493203
Honda M, Kageyama H, Hibino T, Zhang Y, Diono W, Kanda H, Yamaguchi R, Takemura R, Fukaya T, Goto M. Im-proved carotenoid processing with sustainable solvents uti-lizing z-isomerization-induced alteration in physicochemical properties: a review and future directions. Molecules, v. 24, p. 2149, 2019.
https://doi.org/10.3390/molecules24112149
Kaska A, Çiçek M, Mammadov R. Biological activities, phe-nolic constituents and mineral element analysis of two en-demic medicinal plants from Turkey: Nepeta italica subsp. cadmea and Teucrium sandrasicum. South African Journal of Botany, v. 124, p. 63–70, 2019.
https://doi.org/10.1016/j.sajb.2019.04.037
Krishnan S, Salian A, Dutta S, Mandal S. A roadmap to UV-protective natural resources: classification, characteristics, and applications. Materials Chemistry Frontiers, v. 5, p. 7696-7723, 2021.
https://doi.org/10.1039/d1qm00741f
Kultys E, Kurck MA. Green extraction of carotenoids from fruit and vegetable byproducts: a review. Molecules, v. 27, p. 518, 2022. https://doi.org/10.3390/molecules27020518
Lado J, Alós E, Manzi M, Cronje PJR, Gómez-Cadenas A, Rodrigo MJ, Zacarías L. Light regulation of carotenoid bio-synthesis in the peel of mandarin and sweet orange fruits. Frontiers in Plant Science, v. 10, 2019.
https://doi.org/10.3389/fpls.2019.01288
Liew SS, Ho WY, Yeap SK, Sharifudin SAB. Phytochemical composition and in vitro antioxidant activities of Citrus sinensis peel extracts. Peer Journal, v. 6, n. e5331, 2018.
https://doi.org/10.7717/peerj.5331
Makarska-Bialokoz M, Kaczor AA. Computational analysis of chlorophyll structure and UV-Vis spectra: a student research project on the spectroscopy of natural complexes. Spectros-copy Letters, v. 47, n. 2, p. 147-152, 2014.
https://doi.org/10.1080/00387010.2013.781038
Malta LG, Liu RH. Analyses of total phenolics, total flavo-noids, and total antioxidant activities in foods and dietary supplements. Encyclopedia of Agriculture and Food Sys-tems, p. 305–314, 2014.
https://doi.org/10.1016/b978-0-444-52512-3.00058-9
Manthey JA, Grohmann K. Phenols in citrus peel byproducts. Concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses. Journal of Agricultural and Food Chemistry, v. 49, n. 7, p. 3268–3273, 2001.
https://doi.org/10.1021/jf010011r
Maoka T. Carotenoids as natural functional pigments. Journal of Natural Medicine, v. 74, p. 1–16, 2020.
https://doi.org/10.1007/s11418-019-01364-x
Matheyambath AC, Subramanian J, Paliyath G. Mangoes reference module in food science. Encyclopedia of food and health. Switzerland: Elsevier, p. 641–645, 2016.
Mazen FMA, Nashat AA. Ripeness classification of bananas using an Artificial Neural Network. The Arabian Journal for Science and Engineering, v. 44, p. 6901–6910, 2019.
https://doi.org/10.1007/s13369-018-03695-5
Meléndez-Martínez AJ, Mapelli-Brahm P, Hornero-Méndez D, Vicario IM. Structures, nomenclature and general chem-istry of carotenoids and their esters. Carotenoid esters in foods: physical, chemical and biological properties. 2019, 472p.
https://doi.org/10.1039/9781788015851-00001
Melo MMR, Silvestre AJD, Silva CM. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. Jour-nal of Supercritical Fluids, v. 92, p. 115–176, 2014.
https://doi.org/10.1016/j.supflu.2014.04.007
Mensor LL, Menezes FS, Leitão GG, Reis AS, Santos TC, Coube CS, Leitão SG. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytotherapy Research, v. 15, n. 2, p. 127–130, 2001.
https://doi.org/10.1002/ptr.687
Murador DC, Braga ARC, Martins PLG, Mercadante AZ, de Rosso VV. Ionic liquid associated with ultrasonic-assisted extraction: A new approach to obtain carotenoids from or-ange peel. Food Research International, v. 126, 2019.
https://doi.org/10.1016/j.foodres.2019.108653
Nieto G, Fernández-López J, Pérez-Álvarez JA, Peñalver R, Ros-Berruezo G, Viuda-Martos M. Valorization of citrus co-products: recovery of bioactive compounds and applica-tion in meat and meat products. Plants, v. 10, n. 6, p. 1069, 2021.
https://doi.org/10.3390/plants10061069
Njoroge SM, Phi NTL, Sawamura M. Chemical composition of peel essential oils of sweet oranges (Citrus sinensis) from Uganda and Rwanda. Journal of Essential Oil Bearing Plants, v. 12, n. 1, p. 26–33, 2009.
https://doi.org/10.1080/0972060x.2009.10643687
Nur-Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharmaceutical Journal, v. 21, n. 2, p. 143–152, 2013.
https://doi.org/10.1016/j.jsps.2012.05.002
Pereira RM, López BGC, Diniz SN, Antunes AA, Garcia DM, Oliveira CR, Marcucci MC. Quantification of flavo-noids in Brazilian orange peels and industrial orange juice processing wastes. Agricultural Science, v. 8, n. 7, p. 631, 2017.
Pérez-Gálvez A, Viera I, Roca M. Carotenoids and chloro-phylls as antioxidants. Antioxidants, v. 9, n. 6, p. 505, 2020.
https://doi.org/10.3390/antiox9060505
Pérez-Gálvez A., Fontecha J. Analytical protocols in carote-noid analysis. In: Jacob-Lopes E, Queiroz M, Zepka L. Pigments from microalgae handbook. Springer, 2020.
https://doi.org/10.1007/978-3-030-50971-2_7
Pfeil BE, Crisp MD. The age and biogeography of citrus and the orange subfamily (Rutaceae: Aurantioideae) in Australa-sia and New Caledonia. American Journal of Botany, v. 95, n. 12, p. 1621-1631, 2008.
http://dx.doi.org/10.3732/ajb.0800214
Pigni NB, Aranibar C, Mas AL, Aguirre A, Borneo R, Wun-derlin D, Baroni MV. Chemical profile and bioaccessibility of polyphenols from wheat pasta supplemented with partial-ly-deoiled chia flour. LWT-Food Science and Technology, v. 124, 2020.
https://doi.org/10.1016/j.lwt.2020.109134
Pozzan M, Triboni HR. Colheita e qualidade do fruto. Mattos-Junior D, Negri JD, Pio RM, Pompeu-Junior J. Citros. Campinas: Instituto Agronômico/Fundag, 2005. p. 929.
Prieto P, Pineda M, Aguilar M. Spectrophotometric quantita-tion of antioxidant capacity through the formation of a phos-phomolybdenum complex: specific application to the deter-mination of vitamin E. Analytical Biochemistry, v. 269, n. 2, p. 337–341, 1999.
https://doi.org/10.1006/abio.1999.4019
Quelal-Vásconez MA, Lerma-García MJ, Pérez-Esteve E, Talens-Oliag P, Barat-Baviera JM. Roadmap of cocoa quali-ty and authenticity control in the industry: a review of con-ventional and alternative methods. Comprehensive Reviews in Food Science and Food Safety, v. 19, n. 2, p. 448-478, 2020.
https://doi.org/10.1111/1541-4337.12522
Rodrigues MJS, Araújo-Neto SE, Neto RDCA, Soares-Filho WS, Girardi EA, Lessa LS, Araújo JM. Agronomic perfor-mance of the ‘Pera’ orange grafted onto nine rootstocks un-der the conditions of Rio Branco, Acre, Brazil. Revista Bra-sileria de Ciências Agrárias, v. 14, n. 4, p. 1-8, 2019.
Rufino MSM, Alves RE, Brito ES, Pérez-Jiménez J, Saura-Calixto F, Mancini-Filho J. Bioactive compounds and anti-oxidant capacities of 18 nontraditional tropical fruits from Brazil. Food Chemistry, v. 121, n. 4, p. 996-1002, 2010.
http://dx.doi.org/10.1016/jfoodchem.2010.01.037
Sagar S, Goudar G, Sreedhar M, Panghal A, Sharma P. Char-acterization of nutritional content and in vitro antioxidant properties of Plantago ovata seeds. International Journal of Food Sciences and Nutrition, v. 9, p. 27, 2020.
Saguy IS, Sirotinskaya V. Open innovation opportunities focusing on food SMEs. Innovation Strategies in the Food Industry, p. 41-59, 2016.
http://dx.doi.org/10.1016/b978-0-12-803751-5.00003-9
Saini RK, Keum YS. Carotenoid extraction methods: a review of recent developments. Food Chemistry, v. 240, p. 90–103, 2018.
https://doi.org/10.1016/j.foodchem.2017.07.099
Saini RK, Ranjit A, Sharma K, Prasad P, Shang X, Gowda KGM, Keum YS. Bioactive compounds of citrus fruits: a review of composition and health benefits of carotenoids, flavonoids, limonoids, and terpenes. Antioxidants, v. 11, n. 2, p. 239, 2022.
https://doi.org/10.3390/antiox11020239
Sartori JAS, Massarioli AP, Veiga LF, Torres N, Alencar SM, Aguiar CL. Antioxidant capacity of fractions of alco-holic extracts of sugarcane leaves. Zuckerindustrie (Ber-lin)/Sugar Industry, v. 138, p. 165-169, 2013.
https://doi.org/10.36961/si13990
Sawalha SMS, Arráez-Román D, Segura-Carretero A, Fer-nández-Gutiérrez A. Quantification of main phenolic com-pounds in sweet and bitter orange peel using CE–MS/MS. Food Chemistry, v. 116, n. 2, p. 567-574, 2009.
https://doi.org/10.1016/j.foodchem.2009.03.003
Seelam BS, Kuna A, Kavitha C, Kumari MV. Effect of pro-cessing on antioxidant activity of acerola. Research Journal of Agricultural Sciences, v. 7, n. 2, p. 250-255, 2016.
Singh B, Pal-Singh J, Kaur A, Singh N. Phenolic composi-tion, antioxidant potential and health benefits of citrus peel. Food Research International, v. 132, 2020.
https://doi.org/10.1016/j.foodres.2020.109114
Singh P, Shukla R, Prakash B, Kumar A, Singh S, Mishra PK, Dubey NK. Chemical profile, antifungal, antiaflatoxi-genic and antioxidant activity of Citrus maxima Burm and Citrus sinensis (L.) Osbeck essential oils and their cyclic monoterpene, DL-limonene. Food Chemistry and Toxicolo-gy, v. 48, n. 6, p. 1734–1740, 2010.
https://doi.org/10.1016/j.fct.2010.04.001
Spagolla LC, Muniz-Santos M, Passos LL, Aguiar CL. Extra-ção alcoólica de fenólicos e flavonóides totais de mirtilo Ra-bbiteye (Vaccinium ashei) e sua atividade antioxidante. Re-vista de Ciências Farmacêuticas Básica e Aplicada, v. 30, p. 187-191, 2009.
Tiwari S, Upadhyay N, Singh AK, Meena GS, Arora S. Or-ganic solvent-free extraction of carotenoids from carrot bio-waste and its physicochemical properties. Journal of Food Science and Technology, v. 56, p. 4678–4687, 2019.
https://doi.org/10.1007/s13197-019-03920-5
Tsimogiannis D, Oreopoulou V. Classification of phenolic compounds in plants. Polyphenols in Plants, p. 263–284, 2019.
https://doi.org/10.1016/b978-0-12-813768-0.00026-8
Virtanen O, Constantinidou E, Tyystjärvi E. Chlorophyll does not reflect green light: how to correct a misconception. Jour-nal of Biological Education, v. 56, n. 5, p. 552-559, 2020.
https://doi.org/10.1080/00219266.2020.1858930
Vuolo MM, Lima VS, Maróstica-Junior MR. Phenolic com-pounds. Bioactive compounds, p. 33–50, 2019.
https://doi.org/10.1016/b978-0-12-814774-0.00002-5
Wan, C., Yu, Y., Zhou, S., Liu, W., & Tian, S.; Cao, S. Anti-oxidant activity and free radical-scavenging capacity of Gynura divaricata leaf extracts at different temperatures. Pharmacognosy Magazine, v. 7, n. 25, p. 40, 2011.
https://doi:10.4103/0973-1296.75900
Wang Y, Liu XJ, Chen JB, Cao JP, Li X, Sun CD. Citrus flavonoids and their antioxidant evaluation. Critical Reviews in Food Science and Nutrition, p. 1-22, 2021.
https://doi.org/10.1080/10408398.2020.1870035
Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpreta-tion. Nucleic Acids Research, v. 37, p. 652–660, 2009. https://doi.org/10.1093/nar/gkp356
Downloads
Publicado
Como Citar
Edição
Seção
Licença
Copyright (c) 2024 Isabelli Bottene Casagrande, Gabriel Antonio Gianjope Casarotti, Claudio Lima

Este trabalho está licenciado sob uma licença Creative Commons Attribution 4.0 International License.
Copyright (c) 2024 - Journal of Biotechnology and Biodiversity

Este obra está licenciado com uma Licença Creative Commons Atribuição 4.0 Internacional.
Autores que publicam nesta revista concordam com os seguintes termos:
Autores mantêm os direitos autorais e concedem à revista o direito de primeira publicação, com o trabalho simultaneamente licenciado sob a Licença Creative Commons Attribution (CC BY 4.0 no link http://creativecommons.org/licenses/by/4.0/) que permite o compartilhamento do trabalho com reconhecimento da autoria e publicação inicial nesta revista.
Autores têm autorização para assumir contratos adicionais separadamente, para distribuição não exclusiva da versão do trabalho publicada nesta revista (ex.: publicar em repositório institucional ou como capítulo de livro), com reconhecimento de autoria e publicação inicial nesta revista.
Autores têm permissão e são estimulados a publicar e distribuir seu trabalho online (ex.: em repositórios institucionais ou na sua página pessoal) a qualquer momento antes ou durante o processo editorial, já que isso pode gerar alterações produtivas, bem como aumentar o impacto e a citação do trabalho publicado (disponibilizado em O Efeito do Acesso Livre no link http://opcit.eprints.org/oacitation-biblio.html).