Methylene blue dye adsorption study in pink kaolinite
DOI:
https://doi.org/10.20873/jbb.uft.cemaf.v2n3.lealKeywords:
Adsorption, kaolinite, blue methyleneAbstract
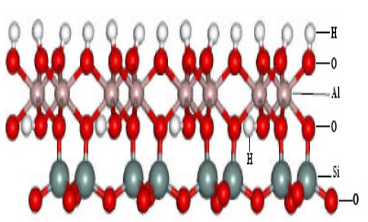
This work aimed to study the adsorption of blue methylene dye using as adsorbent a kind of kaolinite called(named) pink kaolinite. The adsorbent was characterized from its chemical composition, by X-ray diffraction method and surface area by BET method. The variable parameters for the study in question were: the equilibrium time and the ratio of adsorbent mass per volume of adsorbate. It was found that the equilibrium time is less than 20 minutes and the highest adsorption occurred at 1:200 ratio, with a removal of 97%. The isotherm adsorption was constructed from the optimized kinetics adsorption parameters. The data were linearized for the models of Langmuir and Freundlich, and Langmuir model was that better explained the results.
References
Angove, M. J.; Johnson, B. B.; Wells, J. D. (1997), Adsorption of Cadimium (II) on kaolinite. Colloids and Surfaces A, 126, 137-147.
Baldez, E. E.; Robaina, N. F.; Cassella, R. J.(2008), Employment polyurethane foam for the adsorption of methylene blue in aqueous medium. Journal of Hazardous Materials, 159, 580-586.
Baskaralingam, P.; Pulikesi, M.; Ramamurthi, V.; Sivanesan, S. (2007), Modified hectorites and adsorption studies of a reactive dye. Applied Clay Scince, 37, 207-214.
Bharttachayya, K. G. and Gupta, S. S. (2008), Kaolinite and montmorillonite as adsorbents for Fe(III), Co(II) and Ni(II) in aqueous medium.Applied Clay Science, 41, 1-9.
Brady, V. P.; Cygan, T. R.; Nagy, L. K. (1996), Molecular Controls on Kaolinite Surface Charge. Journal of Colloid Interface Scince, 183, 356-364.
Castro, E. A. S. and Martins, J. B. L. (2005), Theoretical study of benzene interaction on kaolinite. Journal of Computer-Aided Materials Design, 12, 121-129.
Guerra D. L.; Souza, J. A.; Airoldi, C.; Viana, R. R. (2008), Avaliação da eficiência de caulinita intercalada com dimetilsulfóxido em adsorção com Zn(II) em meio aquoso – cinética do processo de adsorção. Cerâmica, 54, 273-279.
Harris, R. G.; Wells, J. D.; Johnson, B. B. (2001), Selective adsorption of dyes and other organic molecules to kaolinite and oxide surfaces. Colloids and Surface A, 180, 131-140.
Kunz, A.; Peralta-Zamora, P.; Moraes, S. G.; Durán, N. (2002), Novas tendências no tratamento de efluentes têxteis. Química Nova, 25, 78-82.
Lee,Y. S. and Kim, J. S. (2002), Adsorption of naphthalene by HDTMA modified kaolinite and halloysite. Applied Clay Science, 22, 55-63.
Leja, J. Surface chemistry of froth flotation. New York: Plenum Press, 1982.
Miranda-Trevino, J. C. and Coles, C. A. (2003), Kaolinite properties, structure and influence of metal retention on pH. Applied Clay Scince, 23, 133-139.
Nandi, B. K.; Goswami, A.; Purkait, M. K. (2008), Removal of cationic dyes from aqueous solutions by kaolin: Kinetic and equilibrium studies. Applied Clay Scince, 42, 583-591.
Neumann, M. G; Gessner, F; Cione A. P. P; Sartori A. R; Cavalheiro, S. C. C. (2000), Interações entre corantes e argilas em suspensão aquosa. Química Nova, 23, 818-824.
Pereira, E.; Oliveira, L. C. A.; Vallone, A.; Sapag, K. (2008), Preparação de carvão ativado em baixas temperaturas de carbonização a partir de rejeitos de café: utilização de FeCl3 como agente ativante. Química Nova, 31, 1296-1300.
Roulia, M. and Vassiliadis, A. A. (2008), Sorption characterization of a cationic dye retained by clays and perlite Microporus and Mesoporus Materials, 116, 732-740.
Silva, F. R. A. Avaliação de processosde adsorção de metais pesados: um estudo experimental com propostas de utilização de subprodutos. Dissertação (Mestrado em Sistemas de Gestão) - Universidade Federal Fluminense, 2005.
Tekin, N.; Demirbas, O.; Alkan, M. (2005), Adsorption of cationic polyacrylamide onto kaolinite. Microporous Mesoporous Materials, 85, 340-350.
Turak, F.; Ozgur, M.U.; Afsar, H. (2004), Use of natural and activated clay to remove methylene blue from aqueous solutions. In: 4th AACD Congress, Adnan Menderes University.
Unuabonah, E. I.; Adebowale, K. O.; Dawodu, F. A. (2008), Equilibrium, kinetic and sorber design studies on the adsorption of Aniline blue dye by sodium tetraborate-modified Kaolinite clay adsorbent. Journal of Hazardous Materials, 157, 397-409.
Vijayaraghavan, K.; Won, S. W.; Mao, J.; Yun, Y. (2008), Chemical modification of Corynebacterium glutamicum to improve methylene blue biosorption. Journal of Chemical Engineering, 145, 1-6.
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 - Journal of Biotechnology and Biodiversity

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License (CC BY 4.0 at http://creativecommons.org/licenses/by/4.0/) that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
Authors are permitted and encouraged to post their work online (e.g. in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (Available at The Effect of Open Access, at http://opcit.eprints.org/oacitation-biblio.html).


