Características de promoção do crescimento das plantas em leveduras do solo de ecossistemas naturais brasileiros
DOI:
https://doi.org/10.20873/jbb.uft.cemaf.v13n1.18788Palavras-chave:
ácido indol-3-acético, bioinsumos, fungos unicelulares, solubilização de fosfato, biocontroleResumo
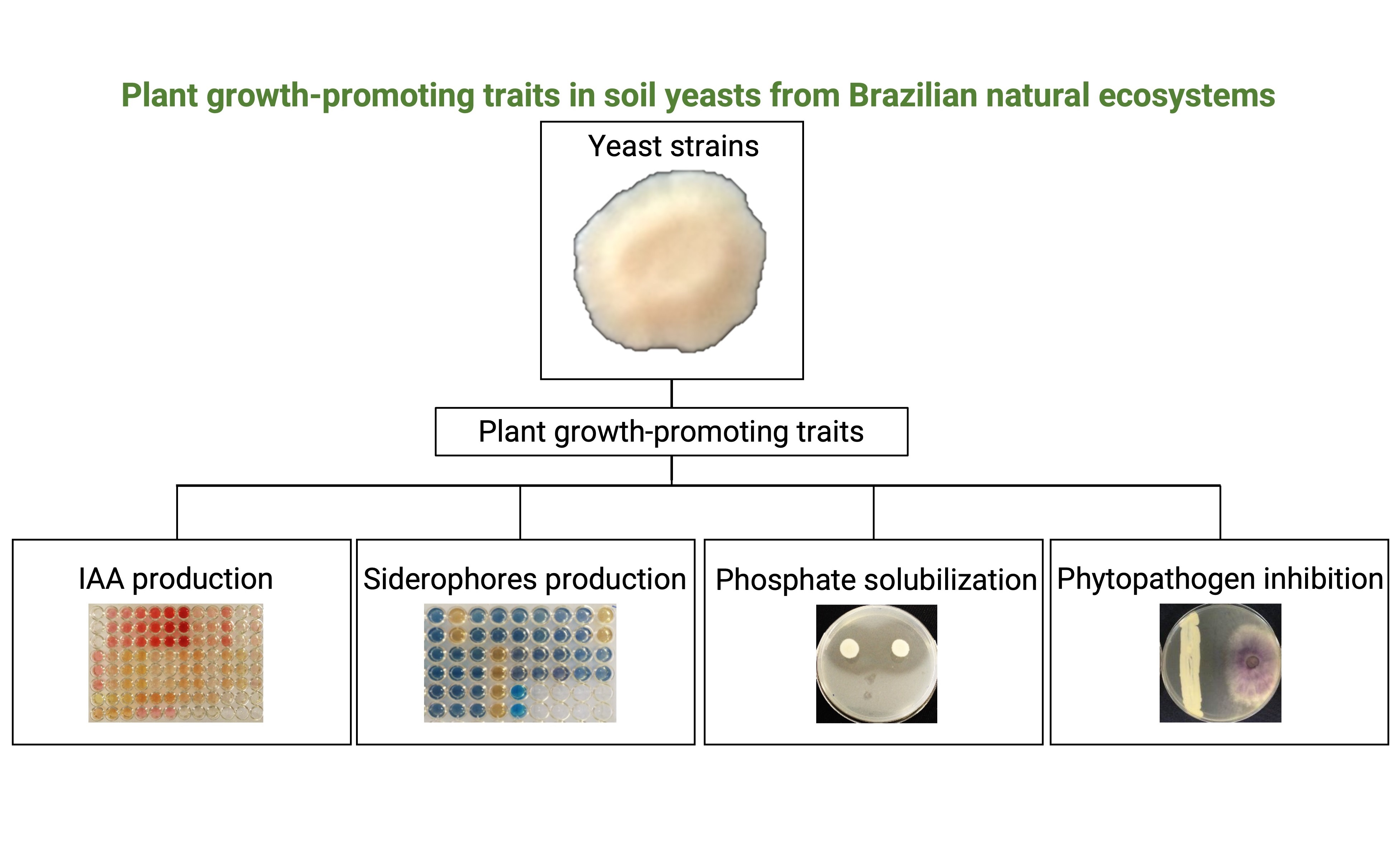
As leveduras do solo são conhecidas por serem habitantes abundantes e diversas nos ecossistemas naturais e também podem desempenhar um papel como promotores do crescimento das plantas. Até onde sabemos, o uso de leveduras de solo de ecossistemas naturais como agentes promotores de crescimento de plantas não foi extensivamente investigado na América do Sul. O objetivo deste estudo foi analisar características de promoção do crescimento vegetal em leveduras de solo de ecossistemas naturais e revegetados. Produção de ácido indol-3-acético foi avaliado em DF meio utilizando L-triptofano como precursor e quantificado pelo método Salkowski. Produção de sideróforos foi avaliada pelo Chrome Azurol S método. Solubilização de fosfato foi avaliada em Pikovskaya agar contendo fosfatos de alumínio e tricálcico. Atividade de biocontrole de fungos fitopatogênicos foi avaliada pelo pareamento de culturas em PDA. No total, 52 isolados apresentaram resultados positivos, representando 17 espécies de leveduras. Rhodotorula spp. foram os melhores produtores de ácido indol-3-acético e capacidade de produção de sideróforos. As espécies Wickerhamomyces anomalus e Meyerozyma guilliermondii apresentaram atividade solubilizadora de fosfato. Oito espécies exibiram efeitos antagônicos contra Fusarium oxysporum. A Candida insectorum, W. anomalus e Rh. mucilaginosa mostraram-se promissoras para investigações futuras. Os resultados do nosso estudo destacam o uso potencial de leveduras do solo como potenciais agentes para produção de bioinsumos visando a produção vegetal.
Referências
Ahmed E, Holmström SJM. Siderophores in environmental research: Roles and applications. Microbial Biotechnology, v. 7, n. 3, p. 196–208, 2014.
https://doi.org/10.1111/1751-7915.12117
Al-Falih AM. Phosphate Solubilization in Vitro By Some Soil Yeasts. Qatar University Sciences Journal, v. 25, p. 119–125, 2005.
Amprayn K, Rose MT, Kecskés M, Pereg L, et al. Plant growth promoting characteristics of soil yeast (Candida tropicalis HY) and its effectiveness for promoting rice growth. Applied Soil Ecology, v. 61, p. 295–299, 2012. https://doi.org/10.1016/j.apsoil.2011.11.009
Berg G. Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorgan-isms in agriculture. Applied Microbiology and Biotechnolo-gy, v. 84, n. 1, p. 11–18, 2009. https://doi.org/10.1007/s00253-009-2092-7
Bernales M, Monsalve L, Ayala-Raso A, Valdenegro M, et al. Expression of two indole-3-acetic acid (IAA)-amido synthe-tase (GH3) genes during fruit development of raspberry (Rubus idaeus Heritage). Scientia Horticulturae, v. 246, p. 168–175, 2019.
https://doi.org/10.1016/j.scienta.2018.09.077
Bispo RLB, Ceccato-Antonini SR, Tosta CD, Fontanetti A, Prado, et al. Sugarcane molasses as substrate to soil yeasts: Indole-3-acetic acid production and maize initial growth promotion. Biocatalysis and Agricultural Biotechnology, v. 47, 2023.
https://doi.org/10.1016/j.bcab.2023.102618
Botha A. The importance and ecology of yeasts in soil. Soil Biology and Biochemistry, v. 43, n. 1, p. 1–8, 2011. https://doi.org/10.1016/j.soilbio.2010.10.001
Bright JP, Karunanadham K, Maheshwari HS, Karuppiah EAA, et al. Seed-Borne Probiotic Yeasts Foster Plant Growth and Elicit Health Protection in Black Gram (Vigna mungo L.). Sustainability, v. 14, n. 4618, 2022. https://doi.org/10.3390/su14084618
Calvente V, Orellano ME, Sansone G, Benuzzi D, Tosetti MIS, et al. Effect of nitrogen source and pH on siderophore production by Rhodotorula strains and their application to biocontrol of phytopathogenic moulds. Journal of Industrial Microbiology and Biotechnology, v. 26, n. 4, p. 226–229, 2001.
doi: 10.1038/sj.jim.7000117.
Chen Y, Xi J, Xiao M, et al. Soil fungal communities show more specificity than bacteria for plant species composition in a temperate forest in China. BMC Microbiology, v. 22, n. 208, 2022.
https://doi.org/10.1186/s12866-022-02591-1
Coda R, Rizzello CG, Cagno RD, Trani A. et al. Antifungal activity of Meyerozyma guilliermondii: Identification of ac-tive compounds synthesized during dough fermentation and their effect on long-term storage of wheat bread. Food Mi-crobiology, v. 33, n. 2, p. 243–251, 2013. https://doi.org/10.1016/j.fm.2012.09.023
de-Bashan LE, Hernandez JP, Bashan Y. The potential contri-bution of plant growth-promoting bacteria to reduce envi-ronmental degradation – A comprehensive evaluation. Ap-plied Soil Ecology, v. 61, p. 171–189, 2012. https://doi.org/10.1016/j.apsoil.2011.09.003
Dell’Amico E, Cavalca L, Andreoni V. Analysis of rhizobac-terial communities in perennial Graminaceae from polluted water meadow soil, and screening of metal-resistant, poten-tially plant growth-promoting bacteria. FEMS Microbiology Ecology, v. 52, p. 153–162, 2006. https://doi.org/10.1016/j.femsec.2004.11.005
El-Mehalawy A. The rhizosphere yeast fungi as biocontrol agents for wilt disease of kidney bean caused by Fusarium oxysporum. International Journal of Agriculture and Biolo-gy, p. 310–316, 2004.
El-Mehalawy AA, Hassanein NM, Youssef YA, Karam Kl-Din, et al. Influence of maize root colonization by the rhizo-sphere actinomycetes and yeast fungi on plant growth and on the biological control of late wilt disease. International Journal of Agriculture and Biology, v. 6, n. 4, p. 599–605, 2004.
El-Tarabily KA, Sivasithamparam K. Potential of yeasts as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Mycoscience, v. 47, n. 1, p. 25–35, 2006.
https://doi.org/10.1007/S10267-005-0268-2
El-Tarabily KA. Suppression of Rhizoctonia solani diseases of sugar beet by antagonistic and plant growth-promoting yeasts. Journal of Applied Microbiology, v. 96, n. 1, p. 69–75, 2004.
https://doi.org/10.1046/j.1365-2672.2003.02043.x
Fernández de Ullivarri M, Mendoza LM, Raya RR. Character-ization of the killer toxin KTCf20 from Wickerhamomyces anomalus, a potential biocontrol agent against wine spoilage yeasts. Biological Control, v. 121, p. 223–228, 2018. https://doi.org/10.1016/j.biocontrol.2018.03.008
Firrincieli A, Otillar R, Salamov A, Schmutz J. et al. Genome sequence of the plant growth promoting endophytic yeast Rhodotorula graminis WP1. Frontiers in Microbiology, v. 6, p. 6–11, 2015.
https://doi.org/10.3389/fmicb.2015.00978
Fu SF, Sun PF, Lu HY, Wei JY, et al. Plant growth-promoting traits of yeasts isolated from the phyllosphere and rhizosphere of Drosera spatulata Lab. Fungal Biology, v. 120, n. 3, p. 433–448, 2016.
https://doi.org/10.1016/j.funbio.2015.12.006
Gastauer M, Souza Filho PWM, Ramos SJ, Caldeira CF, et al. Mine land rehabilitation in Brazil: Goals and techniques in the context of legal requirements. Ambio, v. 1, p. 1-16, 2018. doi: 10.1007/s13280-018-1053-8.
Gordon SA, Weber RP. Colorimetric estimation of indoleace-tic acid. Plant Physiology, v. 26, p. 192–195, 1951. https://doi.org/10.1104/pp.26.1.192
Grzegorczyk M, Żarowska B, Restuccia C, Gabriella Cirvilleri G, et al. Postharvest biocontrol ability of killer yeasts against Monilinia fructigena and Monilinia fructicola on stone fruit. Food Microbiology, v. 61, p. 93–101, 2017. https://doi.org/10.1016/j.fm.2016.09.005
He Y, Hou XY, Li CX, Wang, Y, Ma XR. Soil Microbial Communities Altered by Titanium Ions in Different Agroe-cosystems of Pitaya and Grape. Microbiology Spectrum, v. 10, n. 1, 2022.
https://doi.org/10.1128/spectrum.00907-21
Hesham A, Mohamed H. Molecular genetic identification of yeast strains isolated from egyptian soils for solubilization of inorganic phosphates and growth promotion of corn plants. Journal of Microbiology and Biotechnology, v. 21, p. 55–61, 2011.
https://doi.org/10.4014/jmb.1006.06045
Ignatova LV, Brazhnikova YV, Berzhanova RZ, Mukasheva TD. Plant growth-promoting and antifungal activity of yeasts from dark chestnut soil. Microbiological Research, v. 175, p. 78–83, 2015.
https://doi.org/10.1016/j.micres.2015.03.008
Kaszycki P, Czechowska K, Petryszak P, Miedzobrodzki J, et al. Methylotrophic extremophilic yeast Trichosporon sp.: a soil-derived isolate with potential applications in environ-mental biotechnology. Acta Biochimica Polonica, v. 53, n. 3, p. 463–473, 2006.
Kumla J, Nundaeng S, Suwannarach N, Lumyong S. Evalua-tion of Multifarious Plant Growth Promoting Trials of Yeast Isolated from the Soil of Assam Tea (Camellia sinensis var. assamica) Plantations in Northern Thailand. Microorgan-isms, v. 8, n. 1168, 2020.
https://doi.org/10.3390/microorganisms8081168
Limtong S, Koowadjanakul N. Yeasts from phylloplane and their capability to produce indole-3-acetic acid. World Jour-nal of Microbiology and Biotechnology, v. 28, n. 12, p. 3323–3335, 2012.
https://doi.org/10.1007/s11274-012-1144-9
Liu Bin, Ji S, Zhang H, Wang Y, Liu Z. Isolation of Tricho-derma in the rhizosphere soil of Syringa oblata from Harbin and their biocontrol and growth promotion function. Micro-biological Research, v. 235, 2020. https://doi.org/10.1016/j.micres.2020.126445
Liu W, Wang B, Wang Q, Hou J, et al. Characteristics of metal-tolerant plant growth-promoting yeast (Cryptococcus sp. NSE1) and its influence on Cd hyperaccumulator Sedum plumbizincicola. Environmental Science and Pollution Re-search, v. 23, p. 18621–18629, 2016. https://doi.org/10.1007/s11356-016-7041-2
Manici LM, Caputo F, Castellini M, et al. Binucleate Rhi-zoctonia sp. AG-A, indigenous plant-growth promoting fungus in semi-arid Mediterranean soils. Plant Soil, v. 483, p. 379–393, 2023.
https://doi.org/10.1007/s11104-022-05749-y
Millan A, Fernandez-San I, Farran L, Larraya M, et al. Plant growth-promoting traits of yeasts isolated from Spanish vineyards: benefits for seedling development. Microbiologi-cal Research, v. 237, 2020.
https://doi.org/10.1016/j.micres.2020.126480
Moreira GAM, Mangaravite E, Vieira NM, Silveira FA, et al. Yeast species and strains differing along an altitudinal gradi-ent in the Brazilian forest domain. Revista Brasileira de Ci-ência do Solo, v. 44, 2020.
https://doi.org/ 10.36783/18069657RBCS20200033
Moreira GAM, Vale HMM. Occurrence of yeast species in soils under native and modified vegetation in an iron mining area. Revista Brasileira de Ciência do Solo, v. 42, p. 1–15, 2018.
https://doi.org/10.1590/18069657rbcs20170375
Moreira, GAM, Vale HMM. Soil Yeast Communities in Revegetated Post-Mining and Adjacent Native Areas in Central Brazil. Microorganisms, v. 8, n. 8, 2020. https://doi.org/10.3390/microorganisms8081116
Mukherjee S, Sen SK. Exploration of novel rhizospheric yeast isolate as fertilizing soil inoculant for improvement of maize cultivation. Journal of the Science of Food and Agriculture, v. 95, n. 7, p. 1491-1499, 2014. https://doi.org/10.1002/jsfa.6848
Nakayan, P. et al. Phosphate-solubilizing soil yeast Meyero-zyma guilliermondii CC1 improves maize (Zea mays L.) productivity and minimizes requisite chemical fertilization. Plant and Soil, v. 373, n. 1, p. 301–315, 2013. https://doi.org/10.1007/s11104-013-1792-z
Nassar AH, El-Tarabily KA, Sivasithamparam K. Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biology and Fertility of Soils, v. 42, n. 2, p. 97–108, 2005.
https://doi.org/10.1007/s00374-005-0008-y
Natarajan S, Balachandar D, Senthil N, Velazhahan R, Para-nidharan V. Volatiles of antagonistic soil yeasts inhibit growth and aflatoxin production of Aspergillus flavus. Mi-crobiology Research, v. 263, 2022. https://doi.org/10.1016/j.micres.2022.127150
Nutaratat P, Srisuk N, Arunrattiyakorn P, Limtong S. Plant growth-promoting traits of epiphytic and endophytic yeasts isolated from rice and sugar cane leaves in Thailand. Fungal Biology, v. 118, n. 8, p. 683–694, 2014. https://doi.org/10.1016/j.funbio.2014.04.010
Quadros PD, Zhalnina K, Davis-Richardson AG, Drew JC, et al. Coal mining practices reduce the microbial biomass, rich-ness and diversity of soil. Applied Soil Ecology, v. 98, p. 195-203, 2015.
https://doi.org/10.1016/j.apsoil.2015.10.016
Rafi MM, Krishnaveni MS, Charyulu PBBN. Phosphate-Solubilizing Microorganisms and Their Emerging Role in Sustainable Agriculture. Elsevier Inc. p. 223-233, 2019.
Rao RP, Hunter A, Kashpur O, Normanly J. Aberrant synthe-sis of indole-3-acetic acid in Saccharomyces cerevisiae trig-gers morphogenic transition, a virulence trait of pathogenic fungi. Genetics, v. 185, n. 1, p. 211–220, 2010. doi: 10.1534/genetics.109.112854.
Ribeiro CM, Jurandy E, Nogueira B. Isolation, selection and characterization of root-associated growth promoting bacte-ria in Brazil Pine (Araucaria angustifolia). Microbiological Research, v. 167, n. 2, p. 69–78, 2012. https://doi.org/10.1016/j.micres.2011.03.003
Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant and Soil, v. 321, n. 1, p. 305–339, 2009. https://doi.org/10.1007/s11104-009-9895-2
Saha M, Sarkar S, Sarkar B, Bipin Kumar Sharma BK, et al. Microbial siderophores and their potential applications: a review. Environmental Science and Pollution Research, v. 23, n. 5, p. 3984–3999, 2016.
https://doi.org/10.1007/s11356-015-4294-0
Saharan BS, Nehra V. Plant growth promoting rhizobacteria: a critical review. Life Sciences and Medicine Research, p. 1–30, 2011.
Santos JV, Varón-López M, Soares CRFS, Leal PL, et al. Biological attributes of rehabilitated soils contaminated with heavy metals. Environmental Science and Pollution Re-search, v. 23, n. 7, p. 6735–6748, 2016. https://doi.org/10.1007/s11356-015-5904-6
Sarabia M, Jakobsen I, Grønlund M, Carreon-Abud Y, Larsen J. Rhizospere yeasts improve P uptake of a maize arbuscular mycorrhizal association. Applied Soil Ecology, v. 125, p. 18-25, 2017.
https://doi.org/10.1016/j.apsoil.2017.12.012
Shah S, Shrestha R, Maharjan S, Selosse M-A, Pant B. Isola-tion and characterization of plant growth-promoting endo-phytic fungi from the roots of Dendrobium moniliforme. Plants, v. 8, n. 1, 2018. https://doi.org/10.3390/plants8010005
Silambarasan S, Logeswari P, Cornejo P, Kannan VR. Evalua-tion of the production of exopolysaccharide by plant growth promoting yeast Rhodotorula sp. strain CAH2 under abiotic stress conditions. International Journal of Biological Mac-romolecules, v. 121, p. 55–62, 2019. https://doi.org/10.1016/j.ijbiomac.2018.10.016
Silva AO, Costa AM, Teixeira AFS, Guimarães AA, et al. Soil microbiological attributes indicate recovery of an iron mining area and of the biological quality of adjacent phyto-physiognomies. Ecological Indicators, v. 93, p. 142–151, 2018. https://doi.org/10.1016/j.ecolind.2018.04.073
Sperandio EM, Vale, HMM, Moreira GAM. Yeasts from native Brazilian Cerrado plants: Occurrence, diversity and use in the biocontrol of citrus green mould. Fungal Biology, v. 119, n. 11, p. 984–993, 2015.
https://doi.org/10.1016/j.funbio.2015.06.011
Streletskii RA, Kachalkin AV, Glushakova AM, DeminVV, Chernov IY. Quantitative determination of indole-3-acetic acid in yeasts using high performance liquid chromatog-raphy—tandem mass spectrometry. Microbiology, v. 85, n. 6, p. 727–736, 2016. https://doi.org/10.1134/S0026261716060187
Sun PF, Fang WT, Shin LY, Wei JY, et al. Indole-3-acetic acid-producing yeasts in the phyllosphere of the carnivorous plant Drosera indica L. PLoS ONE, p. 1–22, 2014. https://doi.org/10.1371/journal.pone.0114196
Syed S, Prasad Tollamadugu NVKV. Role of plant growth-promoting microorganisms as a tool for environmental sus-tainability. In Recent Developments in Applied Microbiolo-gy and Biochemistry. Elsevier Inc., p. 209–222, 2019.
Targino HML, Silva VSL, Escobar IEC, Ribeiro PRA, et al. Maize-associated Meyerozyma from the Brazilian semiarid region are effective plant growth-promoting yeasts. Rhizos-phere, v. 22, 2022.
doi.org/10.1016/j.rhisph.2022.100538.
Tenório DA, Medeiros EV, Lima CS, Silva JM, et al. Biologi-cal control of Rhizoctonia solani in cowpea plants using yeast. Tropical Plant Pathology, v. 44, p. 113–119, 2019. https://doi.org/10.1007/s40858-019-00275-2
Thavamani P, Samkumar RA, Satheesh V, Subashchan-drabose SR, et al. Microbes from mined sites: Harnessing their potential for reclamation of derelict mine sites. Envi-ronmental Pollution, v. 230, p. 495–505, 2017. https://doi.org/10.1016/j.envpol.2017.06.056
Yurkov, A.M. Yeasts of the soil - obscure but precious. Yeast, v. 35, n. 5, p. 369–378, 2018. https://doi.org/10.1002/yea.3310
Downloads
Publicado
Como Citar
Edição
Seção
Licença
Copyright (c) 2024 Geisianny Augusta Monteiro Moreira, Helson Mario Martins do Vale

Este trabalho está licenciado sob uma licença Creative Commons Attribution 4.0 International License.
Copyright (c) 2024 - Journal of Biotechnology and Biodiversity

Este obra está licenciado com uma Licença Creative Commons Atribuição 4.0 Internacional.
Autores que publicam nesta revista concordam com os seguintes termos:
Autores mantêm os direitos autorais e concedem à revista o direito de primeira publicação, com o trabalho simultaneamente licenciado sob a Licença Creative Commons Attribution (CC BY 4.0 no link http://creativecommons.org/licenses/by/4.0/) que permite o compartilhamento do trabalho com reconhecimento da autoria e publicação inicial nesta revista.
Autores têm autorização para assumir contratos adicionais separadamente, para distribuição não exclusiva da versão do trabalho publicada nesta revista (ex.: publicar em repositório institucional ou como capítulo de livro), com reconhecimento de autoria e publicação inicial nesta revista.
Autores têm permissão e são estimulados a publicar e distribuir seu trabalho online (ex.: em repositórios institucionais ou na sua página pessoal) a qualquer momento antes ou durante o processo editorial, já que isso pode gerar alterações produtivas, bem como aumentar o impacto e a citação do trabalho publicado (disponibilizado em O Efeito do Acesso Livre no link http://opcit.eprints.org/oacitation-biblio.html).